
In the final blog of our series, we want to share with you reliable information surrounding advanced breast cancer and the available treatments. Nearly a third of people diagnosed with earlier stages of breast cancer will eventually develop advanced disease1,2. Even though many people are living with secondary breast cancer, it can often feel that it is excluded from breast cancer campaigns. Research has shown that women with advanced breast cancer often feel isolated and 67% of them feel like no one understands what they are going through3,4. As part of our campaign this Breast Cancer Awareness Month, we are advocating to increase awareness of all types of breast cancer alongside the treatments available. In this blog, we will be sharing with you accessible treatments for advanced hormone receptor positive (secondary HR+), HER2 negative breast cancer (if you are HER2 positive, head to our previous blog).
What is hormone receptor positive HER2 negative advanced breast cancer?
Secondary breast cancer (also called advanced, metastatic or stage IV) is a breast cancer that has spread beyond the breast and nearby lymph nodes to other parts of the body. Hormone receptor positive (ER positive) HER2 negative metastatic breast cancer indicates that cancer cells grow in response to the hormones oestrogen and progesterone but not in response to the protein HER2. Although it is possible to be initially diagnosed with metastatic breast cancer (called “de novo metastasis”) it is more common to develop metastatic disease after having previously had surgery to treat a localised breast cancer. In fact, a study that looked back at 60,000 women with ER positive localised breast cancer after hormone therapy showed that even some patients with low risk breast cancer (<2 cm tumours, lymph node negative) develop metastatic cancer5. Women that finish hormone therapy five years after having surgery still have a risk of cancer coming back. Therefore, in order to reduce this probability and increase survival, understanding drug resistance, managing side effects so patients can continue treatment and have a great response to therapy is key.
“Something to look forward to is what everyone does. You might have to scale it back from not looking forward to something that is in five years time, but if you can look forward to something that is in three weeks and something that is in three months, and dare to plan it and dare to believe you be able to enjoy it and you will, you will be able to”6 Diane, secondary breast cancer patient
Your treatment options
We have pulled together all the different treatment options for ER positive, HER2 negative metastatic breast cancer that are available on the NHS. We have classified them into four types: hormone therapy, CDK4/6 inhibitors, PIK3/mTOR inhibitors and chemotherapy. In this blog, we are going to identify them all, explain how each work and whether or not they have been approved by the National Institute for Health and Care Excellence (NICE), or by its Scottish counterpart’s, the Scottish Medicines Consortium (SMC) and the Scottish Intercollegiate Guidelines Network (SIGN) which approve new drugs and create guidelines, respectively. If you have locally advanced breast cancer (stage III) where the cancer has spread to the surrounding skin, lymph nodes or chest wall, these treatments will also be available to you.
Hormone therapy
The goal of therapy in this type of cancer is to decrease oestrogen production and/or activity. Thus, all of the hormone therapies look to break the pathway by which oestrogen molecules are produced and interact with breast cancer cells In fact, these therapies can control secondary breast cancer for long periods of time and they are usually the treatment offered as first line therapy.
First line therapy refers to the first treatment given for a disease. If this doesn’t cure the disease or causes severe side effects, then second line therapy would be given, and so forth.
Ovarian suppression
Ovarian suppression is a first line therapy for premenopausal women with secondary breast cancer. Decreasing or stopping ovarian oestrogen production with drugs, such as goserelin (Zoladex®) and Lupron®, or by a surgical removal of the ovaries improves survival7,8.
Aromatase Inhibitors
Aromatase is a protein that converts androgens (created in the adrenal glands) into oestrogens in the fat, liver, muscle or brain cells. Aromatase inhibitors such as Letrozole (Femara®), Anastrozole (Arimidex®) or Exemestane (Aromasin®) inhibit this conversion, decreasing oestrogen levels in the body. These drugs are used in women who are postmenopausal as they improve progression-free survival compared to other therapies such as tamoxifen7,9. 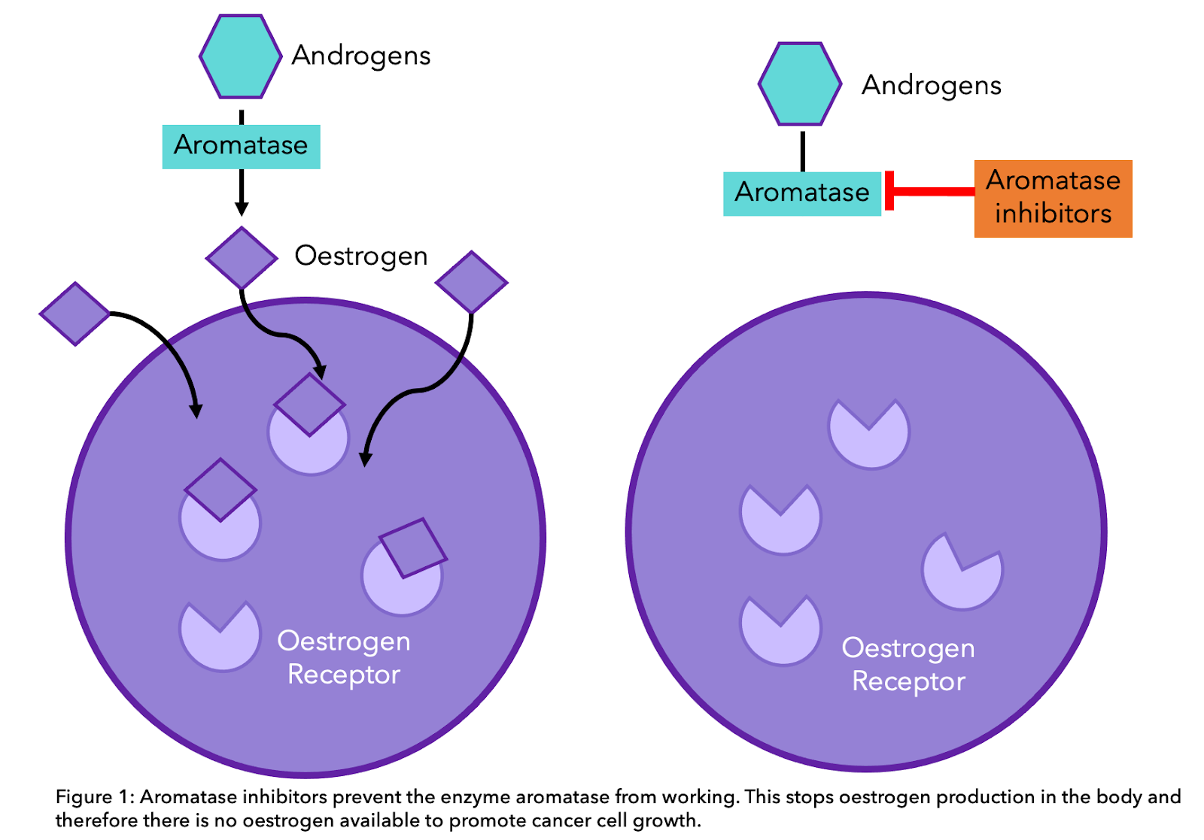
Tamoxifen
Tamoxifen binds directly to the oestrogen receptor (ER) of breast cancer cells, reducing oestrogen activity. It is recommended by NICE along with ovarian suppression for men and premenopausal women10. To know more about tamoxifen and aromatase inhibitors, head to our previous blog. 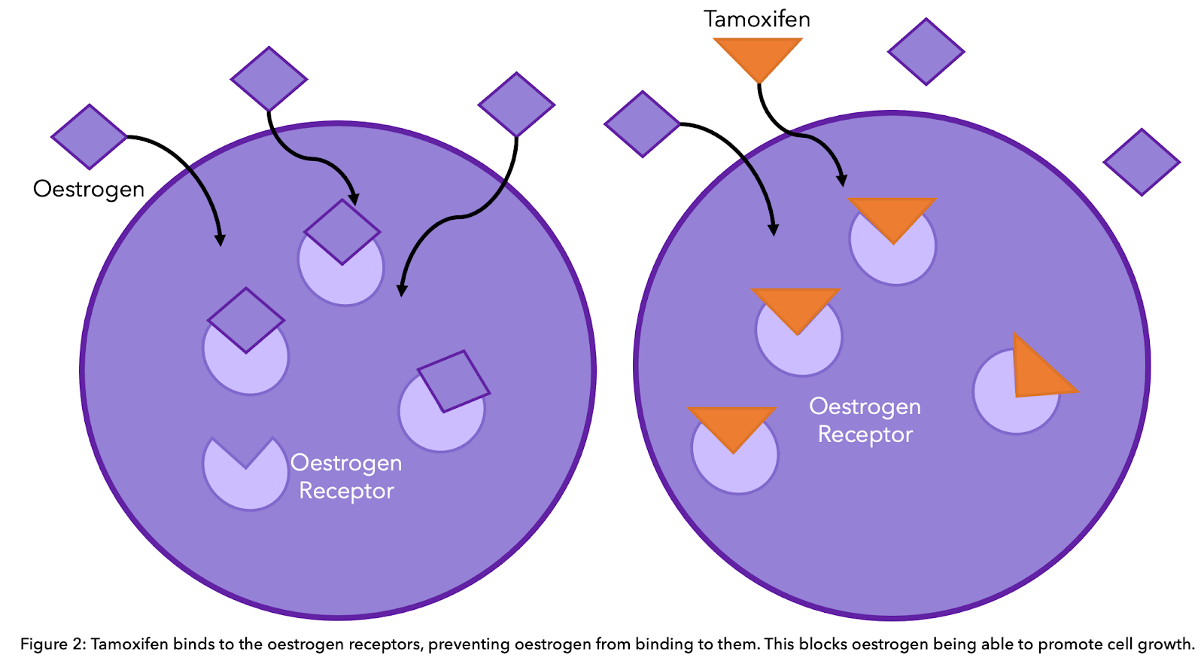
Selective oestrogen receptor degraders (SERDs)
Fulvestrant (Faslodex®)
Fulvestrant (Faslodex®) blocks the oestrogen pathway through a different method to tamoxifen. It binds to the oestrogen receptor (ER) and degrades/breaks this receptor. Therefore, if the oestrogen receptor is degraded, oestrogen cannot bind and drive cancer growth. Fulvestrant is normally offered alongside other therapeutics, such as CDK 4/6 inhibitors and abemaciclib (find out more below), and is not recommended as an alternative to aromatase inhibitors11.
Elacestrant (Orserdu®)
Elacestrant (Orserdu®) is a SERD that can treat ESR1-mutated breast cancer. ESR1 mutations refer to mutations in the oestrogen receptor (ER) gene, which can lead to resistance to hormone treatment (see more below). Elacestrant has been approved as a treatment option in the US and EU to treat secondary Hormone Receptor-positive, HER2-negative, ESR1 mutated breast cancer that has been previously treated with hormone therapy40,52. A diagnostic test is available to identify ESR1 mutations in patients. Elacestrant is not yet a treatment option in the UK, it is currently being reviewed by NICE59.
Why do some patients become resistant to hormone therapy?
Although many people with ER positive breast cancer respond well to hormone therapy, a percentage may become resistant to this treatment after a number of years. Recent studies have looked at the genetics of metastatic ER positive tumours and have found some ways by which cancer cells outsmart hormone therapy. In other words, cancer cells change over time and use alternative pathways to grow. This means that they may no longer be dependent on oestrogen (ER negative) and therefore hormone therapies are not effective. One of the suggested mechanisms points to the oestrogen receptor itself. Mutations in the oestrogen receptor can cause resistance to aromatase inhibitors12,13. Therefore, in cases with ER mutations, some studies suggest that therapies that degrade the receptor can be more effective, such as fulvestrant,14. In fact, patients that become resistant to hormone therapy can benefit from the CDK4/6 inhibitor abemaciclib (described below) in combination with fulvestrant as a second line treatment15. 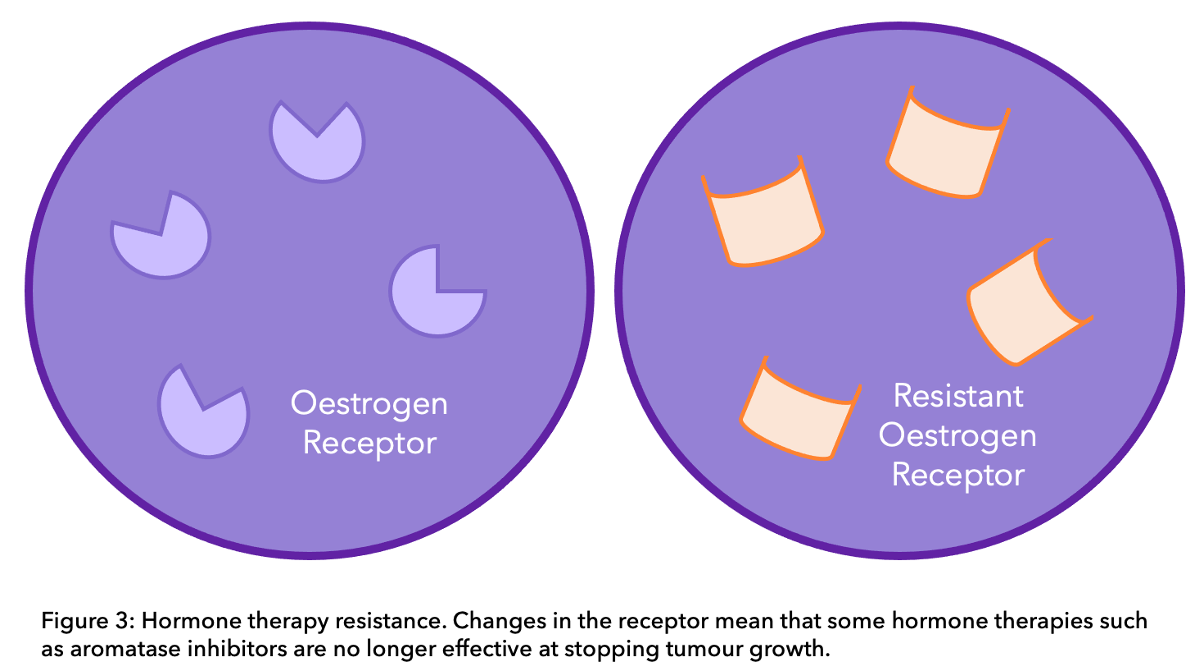
CDK4/6 inhibitors
CDK4/6 inhibitors are a class of oral drugs that inhibit proteins called CDK4 and CDK6. These proteins are important in cell division. Cells have a cell cycle which consists of growing, making DNA (genetic material) and then dividing into more cells. This cell cycle is tightly controlled by many proteins, including CDK4 and CDK6. Therefore, if these proteins are blocked, the cell cycle is interrupted and growth and division are stopped16. Abemaciclib (Verzenio®), palbociclib (Ibrance®) or ribociclib (Kisqali® ) in combination with an aromatase inhibitor are recommended as a first line treatment by NICE for previously untreated metastatic breast cancer7. In fact, clinical trials have demonstrated that these treatments improve survival for around 10 months versus using an aromatase inhibitor alone17,18,19,20. However, currently there are no biomarkers to predict the response to these therapies17. Abemaciclib, palbociclib or ribociclib in combination with fulvestrant can also be prescribed for secondary patients treated previously with hormone therapy (second line therapy) under the Cancer Drug Fund, only if Everolimus combined with exemestane (described below) is the only possible alternative7.
What is the Cancer Drug Fund? The Cancer Drugs Fund is part of the British organisation that reviews new and existing drugs, called NICE. It was made to fund new drugs and accelerate the process of drugs becoming available in England. If there are recently developed treatments that may be of benefit to you, your specialist will fill out an application on your behalf. To find out more about Cancer Drugs Fund, head to Cancer Research UK’s website.
“This is absolutely fantastic news and will now open the door for thousands of women who have received prior hormone therapy to benefit from innovative CDK 4/6 inhibitors, which until now had only been available to newly-diagnosed patients with locally-advanced or metastatic breast cancer.” Baroness Delyth Morgan, Chief Executive at Breast Cancer Care and Breast Cancer Now21.
 CDK4/6 inhibitors can cause a severe decrease in white blood cells (immune cells). Because of this, palbociclib and ribociclib are followed with a seven-day break to allow white blood cell levels to recover. Abemaciclib can be given continuously however fatigue and diarrhea are often experienced. Liver and heart function tests may also be carried out22. Do you know that you can track your side effects with OWise? With over 30 side effects and symptoms to choose from, you can track any changes and share these with your care team and loved ones. Better communication with your care team can make sure you receive the best care possible.
CDK4/6 inhibitors can cause a severe decrease in white blood cells (immune cells). Because of this, palbociclib and ribociclib are followed with a seven-day break to allow white blood cell levels to recover. Abemaciclib can be given continuously however fatigue and diarrhea are often experienced. Liver and heart function tests may also be carried out22. Do you know that you can track your side effects with OWise? With over 30 side effects and symptoms to choose from, you can track any changes and share these with your care team and loved ones. Better communication with your care team can make sure you receive the best care possible.
Other targeted therapies
Everolimus (Afinitor®)
Everolimus is a targeted therapy that blocks the protein mTOR. This protein affects how cells grow and divide. Everolimus with the aromatase inhibitor exemestane is recommended if other treatments such as hormone therapy are ineffective, due to its success in increasing progression-free survival. As with CDK4/6 inhibitors, there are no biomarkers that predict the response to everolimus, so it cannot be determined prior to treatment if it will be effective for your specific breast cancer23. Some of the most common side effects of this therapy are high blood sugar/diabetes, mouth sores, lung inflammation and fatigue. Interestingly, a study found that steroid mouth rinse improves mouth sores from everolimus24. Due to its severe side effects, CDK4/6 inhibitors alongside fulvestrant are the preferred treatment20.
Did you know that Everolimus (Rapamycin) was discovered in Easter Island “Rapa Nui”?25.
Alpelisib (Piqray®)
Alpelisib is a type of targeted therapy that targets a certain protein variation present in some types of breast cancer. Normally the protein, called PI3K, helps all cells get the energy they need. If there is a change, then PI3K works a lot quicker than normal, allowing cancer cells to survive and grow35. The inclusion of alpelisib will depend on the presence of this mutation in your cancer cells. It is thought that 40% of HR+, HER2- breast cancer are positive for this mutation36. A study found that the addition of alpelisib alongside fulvestrant improved overall survival by 8 months34. Alpelisib plus fulvestrant is approved as a treatment option for locally advanced or secondary HR+, HER2- PIK3CA-mutated breast cancer if the cancer has progressed after previous treatment with a CDK4/6 inhibitor plus an aromatase inhibitor37.
Chemotherapy
The most common chemotherapy regimen recommended by the NHS includes both a taxane:
- Paclitaxel (Taxol®), nab-paclitaxel (Abraxane®) or docetaxel (Taxotere®)
And an anthracycline:
- doxorubicin (Adriamycin®), epirubicin (Ellence®), doxorubicin (Doxil®), daunorubicin (Cerubidine®) or mitoxantrone (Novantrone®).
For patients who are not suitable for anthracyclines, docetaxel is offered as first-line treatment, followed by vinorelbine or capecitabine7. Gemcitabine can be used when it is combined with paclitaxel, and after other chemotherapy medicines like anthracyclines have either not worked or were considered unsuitable7. During the COVID-19 pandemic, chemotherapy regimens have slightly changed. Some common intravenous chemotherapy drugs (e.g Taxol®) are being replaced by oral chemotherapy pills such as capecitabine (Xeloda®), and Abraxane® might be prioritised over Taxol® due to its reduced toxicity (fewer side effects) (if you want to understand the difference between Taxol and Abraxane, head to this Instagram post)26.
After chemotherapy: Eribulin
The chemotherapy drug eribulin is recommended after two or more chemotherapy regimens with anthracycline or taxane, and capecitabine. The most common side effects of this therapy are hair loss, nausea, low white cell counts, fatigue and numbness or tingling7.
Did you know that eribulin (aka Halaven®) was created using a sea sponge?
It’s modelled from halichondrin B, which was first discovered in 1986 in marine sponges. Due to its complexity and the lack of it naturally, a synthetic version was made.
Antibody-drug conjugates (ADCs)
Antibody-drug conjugates are highly targeted innovative treatments made up of a monoclonal antibody linked to a chemotherapy drug. The monoclonal antibody component binds to a specific target on cancer cells. This allows the chemotherapy drug to be delivered directly to cancer cells and destroy them, while having minimal impact on healthy cells.
Sacituzumab govitecan (Trodelvy®)
Sacituzumab govitecan (Trodelvy®) is made up of the monoclonal antibody sacituzumab, linked to the chemotherapy drug SN-38. Sacituzumab targets the TROP-2 protein, which is a new protein found in abundance in triple negative breast cancer. It has recently been found that TROP-2 is often present in high levels on HR+ breast cancer cells, which led to anti-TROP-2 therapy being tested in secondary HR+, HER2- breast cancer patients in the TROPiCS-02 trial41. Primary results led to approval in the US and EU for sacituzumab govitecan to treat secondary HR+, HER2- breast cancer that has been previously treated with hormone therapy and at least two other lines of treatment for metastatic disease42. It is currently being reviewed by NICE and may become a treatment option in the UK in the future.
Trastuzumab deruxtecan (Enhertu®)
Trastuzumab deruxtecan (Enhertu®) is made up of the monoclonal antibody trastuzumab linked to the chemotherapy drug deruxtecan. Trastuzumab targets the HER2 protein on the surface of cancer cells. The DESTINY-Breast04 trial studied trastuzumab deruxtecan in HER2-low patients, defined by an immunohistochemistry (ICH) score of 1+ or an IHC 2+ with a negative ISH test44. This accounts for around 45-55% of all breast cancer patients45. Trastuzumab deruxtecan is approved in the US and EU as a treatment option for HER2-low patients that have been previously treated with chemotherapy for metastatic disease or have developed a recurrence during or within 6 months of completing chemotherapy after surgery46,47. Trastuzumab deruxtecan is currently being reviewed by NICE to treat HER2-low patients and a decision is expected in November 2023.
PARP inhibitors
PARP inhibitors are a type of treatment that can be offered to people who are BRCA positive. The most well known of these, olaparib (Lynparza®), is approved to treat secondary HER2-, BRCA+ breast cancer patients after chemotherapy in the US and EU48,49. Talazoparib (Talzenna®) is also approved in the US and EU to treat secondary HER2-, BRCA+ breast cancer50,51. Both treatments are under review by NICE, the olaparib appraisal is on hold as NICE are waiting for the manufacturer to submit new data and a decision about talazoparib is expected later in 2023.
Metastases
Bone metastases
If your breast cancer has spread to your bones, there are an array of treatments that you could be offered for both preventing fractures and other bone-related problems, as well as pain management.
Bisphosphonates and Denosumab
There are multiple bisphosphonates available, namely zoledronic acid (Zometa®) and ibandronate (Bondronat®), which can be offered either orally or through injections27. They act through targeting the cells that break down old bone. In bone metastases, where there is already a deficit of healthy bone cells, bisphosphonates stopping bone cell breakdown can make the bone denser and less likely to fracture28. Denosumab is a type of targeted therapy called a monoclonal antibody (find out how monoclonal antibodies work in our blog). These can be offered instead of bisphosphonates, even though their function is the same. Rather than being absorbed by the cells that break down bone, as bisphosphonates are, denosumab targets a protein that controls the cells. A review of denosumab found that it was more clinically effective than bisphosphonates in terms of the length of time before skeletal-related events occurred. There were also less side effects in the kidneys. As denosumab is administered through an injection under the skin, it decreases the number of trips needed to the hospital27.
Brain metastases
Whole brain radiotherapy will be offered to people who have a single or small number of metastases in their brain that could be resectable and to those whom surgery is not appropriate29.
Pain relief
There are accessible treatments that have been shown to effectively control pain during secondary breast cancer. You will initially be given mild pain relief which can then develop to moderate and then strong pain relief when necessary. Morphine-based pain relief has been shown to be effective in controlling many types of pain. Morphine is taken through oral pills, patches or injections under the skin30. Some of the most common morphine side effects include sleepiness, constipation and nausea. It is also important to remember that it is illegal to drive when using morphine. Interestingly, this is not the case in Scotland31. Doctors often use several other types of drugs to help manage pain. These include:
- Anti-inflammatory drugs, such as ibuprofen, diclofenac or naproxen steroids.
- Anti-depression and epilepsy drugs, which can also help relieve certain types of pain.
Future treatments
Treatment for advanced ER positive breast cancer is constantly developing. With better understanding of the biology of cancer cells, new therapies can be created. Here we have listed the therapies that are currently being studied.
Advanced Genomic Profiling
In breast cancer, treatment options are decided depending on the presence of oestrogen receptors or HER2 on breast cancer cells. This does not highlight the changes from tumour evolution, causing cases such as the hormone therapy resistance described earlier. To allow for more targeted therapies, advanced genomic profiling provides a more in-depth understanding of your breast cancer type. Through rigorously searching your DNA, changes in genes are identified that could make you eligible for different treatments or clinical trials. Certain changes in genes can make you eligible for a specific treatment. An example of this is in the gene PIK3CA, which makes you eligible for alpelisib (see above). Other findings are not as direct as these, and it is the accruing of various factors that will help decide the best treatment option for you. If you are not responding to your current treatment, we suggest researching the potential of genomic profiling. Although unavailable on the NHS at present, there are multiple companies that provide the service which can be refundable through healthcare insurance. It is vital to note that you should discuss this with your oncologist. Together you can decide whether it could improve your treatment.
Companies providing advanced genomic profiling:
Capivasertib
Capivasertib is an AKT inhibitor that is currently being studied in a phase III clinical trial. It works by targeting the AKT pathway, thereby stopping cancer growth. Capivasertib is being tested in secondary HR+, HER2- patients with progression or recurrence after treatment with an aromatase inhibitor. They must also have been treated with chemotherapy for metastatic disease. Additionally, capivasertib is being tested in tumours with mutations (changes) in certain genes in the AKT pathway, including PIK3CA, AKT1 or PTEN genes.
Results from the CAPItello-291 trial showed that in the overall population most patients on capivasertib and fulvestrant went on without the cancer growing or spreading for 7.2 months on average, compared to 3.6 months on placebo plus fulvestrant53. In patients with mutations in the AKT pathway most went on without the cancer growing or spreading for 7.3 months on average, compared to 3.1 months on placebo plus fulvestrant53. Capivasertib is not yet approved, but in June 2023 it was granted for priority review by the FDA and may become a treatment option in the future54.
“This Priority Review decision underscores the potential of capivasertib to extend the effectiveness of endocrine-based treatment approaches for patients with HR-positive breast cancer who experience tumor progression on, or resistance to these widely used therapies. We look forward to working with the FDA to bring this potential first-in-class AKT inhibitor to patients as quickly as possible.” – Susan Galbraith, Executive Vice President, Oncology R&D, AstraZeneca54.
Camizestrant
Camizestrant is a next generation SERD that breaks down the oestrogen receptor signalling pathway through binding to and degrading oestrogen receptors on cancer cells. It is currently in phase II clinical trials being tested in post-menopausal HR+, HER2- secondary breast cancer patients that have been previously treated with hormone therapy for metastatic disease. The SERENA-2 trial found that most patients taking 75 mg of camizestrant per day went on without the cancer growing or spreading for 7.2 months on average, compared to 3.7 months on fulvestrant alone55. There are also two ongoing phase III clinical trials, SERENA-4 and SERENA-6, that are evaluating camizestrant in combination with a CDK 4/6 inhibitor56,57.
Datopotamab deruxtecan
Datopotamab deruxtecan is a new antibody-drug conjugate that is in an ongoing phase III clinical trial. Similarly to sacituzumab govitecan (Trodelvy®), the monoclonal antibody datopotamab targets the TROP-2 protein on the surface of cancer cells. The TROPION-Breast01 trial looked at this drug in secondary or inoperable HR+, HER2-low, or HER2- breast cancer that had been previously treated with hormone therapy and at least one other type of therapy. AstraZeneca announced that the first results from the TROPION-Breast01 trial showed that datopotamab deruxtecan significantly improved the amount of time patients lived without the cancer growing or spreading, compared to patients on chemotherapy alone58. The data is being submitted to country specific regulatory bodies for review.
Combined Immunotherapy
Cancer immunotherapy, which includes monoclonal antibodies such as Herceptin, have been successful in improving survival, however its success have been limited to subsets of the population. MORPHEUS trial is currently studying the effect of combining immunotherapy with chemotherapy or targeted therapy. This is using the drug atezolizumab, a monoclonal antibody, used in triple negative breast cancer and secondary HR+ breast cancer38.
HDAC inhibitors
A new method of targeting individuals specific breast cancer cells is through inhibiting histone deacetylase. The treatment (HDAC’s), including tucidinostat and entinostat, block the function of histone deacetylase, a protein vital in the process of cell growth. Tucidinostat was found to significantly improve progression-free survival compared to having the aromatase inhibitor exemestane alone. This could pose as a new treatment options for postmenopausal women with advanced breast cancer who have not responded to hormone therapy. However, adverse side effects were noted in 50% of the patients given the drug, and therefore further research is necessary39.
And that’s all the treatments summed up for secondary HR+ breast cancer.
We hope that you now better understand your treatment options regarding secondary HR+ breast cancer and can feel confident in discussions with your care team. Our aim is to make sure you are kept informed so make sure to follow our Instagram and Twitter accounts for any updates. If you would like access to more personalised information, download OWise today.
References
- O’Shaughnessy, J. (2005). Extending Survival with Chemotherapy in Metastatic Breast Cancer. The Oncologist, 10(suppl_3), pp.20–29.
- www.cancer.org. (n.d.). Advanced and Metastatic Cancer. [online] Available at: https://www.cancer.org/treatment/understanding-your-diagnosis/advanced-cancer.html.
- Results of the 2014 Realities of living with ABC survey commissioned by Novartis Oncology in the UK with 60 respondents living with advanced breast cancer distributed by Breast Cancer Care.
- Results of The Count Us, Know Us, Join Us survey 2013, commissioned by Novartis Oncology in 12 countries with nearly 1 300 total respondents living with advanced breast cancer including 79 in the UK. Conducted by Harris Interactive.
- Pan, H., Gray, R., Braybrooke, J., Davies, C., Taylor, C., McGale, P., Peto, R., Pritchard, K.I., Bergh, J., Dowsett, M. and Hayes, D.F. (2017). 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. New England Journal of Medicine, [online] 377(19), pp.1836–1846. Available at: https://www.nejm.org/doi/full/10.1056/NEJMoa1701830.
- Breast Cancer Now. (2018). Secondary breast cancer diagnosis. [online] Available at: https://breastcancernow.org/information-support/facing-breast-cancer/secondary-metastatic-breast-cancer/secondary-breast-cancer-diagnosis [Accessed 26 Oct. 2020].
- pathways.nice.org.uk. (7AD). Advanced breast cancer – NICE Pathways. [online] Available at: https://pathways.nice.org.uk/pathways/advanced-breast-cancer [Accessed 26 Oct. 2020].
- Taylor, C.W., Green, S., Dalton, W.S., Martino, S., Rector, D., Ingle, J.N., Robert, N.J., Budd, G.T., Paradelo, J.C., Natale, R.B., Bearden, J.D., Mailliard, J.A. and Osborne, C.K. (1998). Multicenter randomized clinical trial of goserelin versus surgical ovariectomy in premenopausal patients with receptor-positive metastatic breast cancer: an intergroup study. Journal of Clinical Oncology, 16(3), pp.994–999.
- Mouridsen, H., Gershanovich, M., Sun, Y., Pérez-Carrión, R., Boni, C., Monnier, A., Apffelstaedt, J., Smith, R., Sleeboom, H.P., Jaenicke, F., Pluzanska, A., Dank, M., Becquart, D., Bapsy, P.P., Salminen, E., Snyder, R., Chaudri-Ross, H., Lang, R., Wyld, P. and Bhatnagar, A. (2003). Phase III Study of Letrozole Versus Tamoxifen as First-Line Therapy of Advanced Breast Cancer in Postmenopausal Women: Analysis of Survival and Update of Efficacy From the International Letrozole Breast Cancer Group. Journal of Clinical Oncology, 21(11), pp.2101–2109.
- Nice.org.uk. (2009). Recommendations | Advanced breast cancer: diagnosis and treatment | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/cg81/chapter/recommendations [Accessed 26 Oct. 2020].
- www.nice.org.uk. (n.d.). 1 Recommendations | Fulvestrant for untreated locally advanced or metastatic oestrogen-receptor positive breast cancer | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/TA503/chapter/1-Recommendations [Accessed 26 Oct. 2020].
- Chandarlapaty, S., Chen, D., He, W., Sung, P., Samoila, A., You, D., Bhatt, T., Patel, P., Voi, M., Gnant, M., Hortobagyi, G., Baselga, J. and Moynahan, M.E. (2016). Prevalence of ESR1 Mutations in Cell-Free DNA and Outcomes in Metastatic Breast Cancer. JAMA Oncology, 2(10), p.1310.
- Razavi, P., Chang, M.T., Xu, G., Bandlamudi, C., Ross, D.S., Vasan, N., Cai, Y., Bielski, C.M., Donoghue, M.T.A., Jonsson, P., Penson, A., Shen, R., Pareja, F., Kundra, R., Middha, S., Cheng, M.L., Zehir, A., Kandoth, C., Patel, R., Huberman, K., Smyth, L.M., Jhaveri, K., Modi, S., Traina, T.A., Dang, C., Zhang, W., Weigelt, B., Li, B.T., Ladanyi, M., Hyman, D.M., Schultz, N., Robson, M.E., Hudis, C., Brogi, E., Viale, A., Norton, L., Dickler, M.N., Berger, M.F., Iacobuzio-Donahue, C.A., Chandarlapaty, S., Scaltriti, M., Reis-Filho, J.S., Solit, D.B., Taylor, B.S. and Baselga, J. (2018). The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell, 34(3), pp.427-438.e6.
- Fribbens, C., O’Leary, B., Kilburn, L., Hrebien, S., Garcia-Murillas, I., Beaney, M., Cristofanilli, M., Andre, F., Loi, S., Loibl, S., Jiang, J., Bartlett, C.H., Koehler, M., Dowsett, M., Bliss, J.M., Johnston, S.R.D. and Turner, N.C. (2016). Plasma ESR1 Mutations and the Treatment of Estrogen Receptor-Positive Advanced Breast Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology, [online] 34(25), pp.2961–8. Available at: https://www.ncbi.nlm.nih.gov/pubmed/27269946/ [Accessed 26 Oct. 2020].
- www.royalmarsden.nhs.uk. (n.d.). Two studies co-led by The Royal Marsden and presented at this year’s ASCO investigated the use of abemaciclib for patients with advanced metastatic breast cancer. | The Royal Marsden NHS Foundation Trust. [online] Available at: https://www.royalmarsden.nhs.uk/two-studies-co-led-royal-marsden-and-presented-years-asco-investigated-use-abemaciclib-patients [Accessed 26 Oct. 2020].
- Breastcancer.org. (2020). CDK4/6 Inhibitors for Metastatic Breast Cancer: Ibrance, Kisqali, Verzenio. [online] Available at: https://www.breastcancer.org/treatment/targeted_therapies/cdk46-inhibitors.
- Turner, N.C., Slamon, D.J., Ro, J., Bondarenko, I., Im, S.-A., Masuda, N., Colleoni, M., DeMichele, A., Loi, S., Verma, S., Iwata, H., Harbeck, N., Loibl, S., André, F., Puyana Theall, K., Huang, X., Giorgetti, C., Huang Bartlett, C. and Cristofanilli, M. (2018). Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. New England Journal of Medicine, [online] 379(20), pp.1926–1936. Available at: https://www.nejm.org/doi/full/10.1056/NEJMoa1810527.
- Im, S.-A., Lu, Y.-S., Bardia, A., Harbeck, N., Colleoni, M., Franke, F., Chow, L., Sohn, J., Lee, K.-S., Campos-Gomez, S., Villanueva-Vazquez, R., Jung, K.-H., Chakravartty, A., Hughes, G., Gounaris, I., Rodriguez-Lorenc, K., Taran, T., Hurvitz, S. and Tripathy, D. (2019). Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. New England Journal of Medicine, [online] 381(4), pp.307–316. Available at: https://cdn.mednet.co.il/2019/10/2019-LEE-BC-Im-et-al-ML-7-OS-NEJM-2019.pdf
- Johnston, S., Martin, M., Di Leo, A., Im, S.-A., Awada, A., Forrester, T., Frenzel, M., Hardebeck, M.C., Cox, J., Barriga, S., Toi, M., Iwata, H. and Goetz, M.P. (2019). MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. npj Breast Cancer, 5(1).
- Sledge, G.W., Toi, M., Neven, P., Sohn, J., Inoue, K., Pivot, X., Burdaeva, O., Okera, M., Masuda, N., Kaufman, P.A., Koh, H., Grischke, E.-M., Conte, P., Lu, Y., Barriga, S., Hurt, K., Frenzel, M., Johnston, S. and Llombart-Cussac, A. (2020). The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor–Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2. JAMA Oncology, 6(1), p.116.
- Breast Cancer Now. (2019). We respond to NICE approval of abemaciclib with fulvestrant for use on the Cancer Drugs Fund. [online] Available at: https://breastcancernow.org/about-us/media/statements/we-respond-nice-approval-abemaciclib-fulvestrant-use-cancer-drugs-fund [Accessed 26 Oct. 2020].
- Breast Cancer Now. (2018). Palbociclib (Ibrance). [online] Available at: https://breastcancernow.org/information-support/facing-breast-cancer/going-through-breast-cancer-treatment/targeted-therapy/palbociclib-ibrance [Accessed 26 Oct. 2020].
- Baselga, J., Campone, M., Piccart, M., Burris, H.A., Rugo, H.S., Sahmoud, T., Noguchi, S., Gnant, M., Pritchard, K.I., Lebrun, F., Beck, J.T., Ito, Y., Yardley, D., Deleu, I., Perez, A., Bachelot, T., Vittori, L., Xu, Z., Mukhopadhyay, P., Lebwohl, D. and Hortobagyi, G.N. (2012). Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. New England Journal of Medicine, 366(6), pp.520–529.
- Rugo, H.S., Seneviratne, L., Beck, J.T., Glaspy, J.A., Peguero, J.A., Pluard, T.J., Dhillon, N., Hwang, L.C., Nangia, C., Mayer, I.A., Meiller, T.F., Chambers, M.S., Sweetman, R.W., Sabo, J.R. and Litton, J.K. (2017). Prevention of everolimus-related stomatitis in women with hormone receptor-positive, HER2-negative metastatic breast cancer using dexamethasone mouthwash (SWISH): a single-arm, phase 2 trial. The Lancet Oncology, 18(5), pp.654–662.
- Seto, B. (2012). Rapamycin and mTOR: a serendipitous discovery and implications for breast cancer. Clinical and Translational Medicine, 1(1), p.29.
- Nice.org.uk. 2020. NHS England interim treatment options during theCOVID-19 pandemic [online]. Available at: https://www.nice.org.uk/guidance/ng161/resources/interim-treatment-change-options-during-the-covid19-pandemic-endorsed-by-nhs-england-pdf-8715724381
- www.nice.org.uk. (n.d.). 4 Evidence and interpretation | Denosumab for the prevention of skeletal-related events in adults with bone metastases from solid tumours | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/ta265/chapter/4-Evidence-and-interpretation [Accessed 26 Oct. 2020b].
- www.cancerresearchuk.org. (n.d.). How bisphosphonates work | Cancer Research UK. [online] Available at: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/bisphosphonates/how-bisphosphonates-work [Accessed 26 Oct. 2020].
- www.nice.org.uk. (n.d.). Recommendations | Advanced breast cancer: diagnosis and treatment | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/cg81/chapter/Recommendations#managing-complications [Accessed 26 Oct. 2020c].
- Nice.org.uk. (2012). Overview | Palliative care for adults: strong opioids for pain relief | Guidance | NICE. [online] Available at: https://www.nice.org.uk/Guidance/CG140.
- Breast Cancer Now. (2015). Pain control and secondary breast cancer. [online] Available at: https://breastcancernow.org/information-support/facing-breast-cancer/secondary-metastatic-breast-cancer/pain-control-secondary-breast-cancer [Accessed 26 Oct. 2020].
- Bardia, A. et al., (2018) Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (secondary HR+)/HER2- metastatic breast cancer (mBC). Journals of Clinical Oncology. 35(15):suppl, 1004-1004
- Clinicaltrials.gov. (2020). Study of IMMU-132 in secondary HR+/HER2- MBC (TROPICS-02). [online] Available at: https://clinicaltrials.gov/ct2/show/NCT03901339 [Accessed 26 Oct. 2020].
- André. F. (2020). LBA18 – Overall survival (os) results from SOLAR-1, a phase III study of alpelisib (ALP) + fulvestrant (FUL) for hormone receptor-positive (secondary HR+), human epidermal growth factor receptor 2-negative (HER2–) advanced breast cancer (ABC). Annals of Oncology. 31 (suppl_4): S1142-S1215
- Breastcancer.org.(2020). Piqray. [online] Available at: .https://www.breastcancer.org/treatment/targeted_therapies/piqray [Accessed 26 Oct. 2020].
- André, F. et al,. (2019). Alpelisib For PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. New England Journal Of Medicine. 380:1929-1940.
- www.nice.org.uk. (n.d.).Alpelisib with fulvestrant for treating hormone-receptor positive, HER2-negative, PIK3CA-positive advanced breast cancer [online] Available at: https://www.nice.org.uk/guidance/TA652 [Accessed 14 July. 2022].
- Yardley DA, Abu-Khalaf M, Boni V, Brufsky A, Emens LA, Gutierrez M, Hurvitz S, Im S-A, Loi S, McCune SL, Schmid P, O’Hear C, Zhang X, Vidal GA. (2019). MORPHEUS: A phase Ib/II trial platform evaluating the safety and efficacy of multiple cancer immunotherapy combinations in patients with hormone receptor–positive and triple-negative breast cancer [abstract]. In: Proceedings of the 2018 San Antonio Breast Cancer Symposium; 2018 Dec 4-8; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 79(4 Suppl):Abstract nr OT2-06-04.
- Jiang, Zefei et al. (2019). Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology. 20(6):806-815
- www.FDA.gov. (2023). FDA approves elacestrant for ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-elacestrant-er-positive-her2-negative-esr1-mutated-advanced-or-metastatic-breast-cancer
- Rugo HS, Bardia A, Marmé F, Cortes J, Schmid P, Loirat D, Tredan O, Ciruelos E, Dalenc F, Gómez Pardo P, et al. (2022). Primary results from TROPiCS-02: A randomized phase 3 study of sacituzumab govitecan (SG) versus treatment of physician’s choice (TPC) in patients (Pts) with hormone receptor–positive/HER2-negative (HR+/HER2-) advanced breast cancer. Journal of Clinical Oncology. 2022;40(17_suppl):LBA1001–LBA1001.
- www.FDA.gov. (2023). FDA approves sacituzumab govitecan-hziy for HR-positive breast cancer. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-sacituzumab-govitecan-hziy-hr-positive-breast-cancer
- www.EMA.eu. (2021). Trodelvy – European Medicines Agency. European Medicines Agency. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/trodelvy
- Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, et al. (2023). Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. New England Journal of Medicine. 387(1).
- Li Y, Tsang J, Tam F, Loong T, Tse G. (2023). Comprehensive characterization of HER2-low breast cancers: implications in prognosis and treatment. eBioMedicine. 91:104571.
- www.FDA.gov. (2022). FDA Approves First Targeted Therapy for HER2-Low Breast Cancer. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer
- www.EMA.eu. (2020). Enhertu – European Medicines Agency. European Medicines Agency. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/enhertu
- www.FDA.gov. (2019). FDA approves olaparib for germline BRCA-mutated metastatic breast canc. FDA. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-germline-brca-mutated-metastatic-breast-cancer
- www.EMA.eu. Olaparib – ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Available at: https://www.ema.europa.eu/en/documents/product-information/lynparza-epar-product-information_en.pdf
- www.FDA.gov. (2019). FDA approves talazoparib for gBRCAm HER2-negative locally advanced or metastatic breast cancer. FDA. Available at: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-gbrcam-her2-negative-locally-advanced-or-metastatic-breast-cancer
- www.EMA.eu. Talazoparib – ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS. Available at: https://www.ema.europa.eu/en/documents/product-information/lynparza-epar-product-information_en.pdf
- Cancer Network. (2023). Elacestrant Earns EU Approval for ESR1+, ER+, HER2– Advanced Breast Cancer. Cancer Network. Available at: https://www.cancernetwork.com/view/elacestrant-earns-eu-approval-for-esr1-er-her2-advanced-breast-cancer
- Turner NC, Oliveira M, Howell SJ, Dalenc F, Cortes J, Gomez Moreno HL, Hu X, Jhaveri K, Krivorotko P, Loibl S, et al. (2023). Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. The New England Journal of Medicine. 388(22):2058–2070.
- AstraZeneca. (2023). Capivasertib in combination with Faslodex granted Priority Review in the US for patients with advanced HR-positive breast cancer. AstraZeneca. Available at: https://www.astrazeneca.com/media-centre/press-releases/2023/capivasertib-in-combination-with-faslodex-granted-priority-review-in-the-us.html
- Helwick C. (2023). Camizestrant Improves Progression-Free Survival in Advanced Breast Cancer – The ASCO Post. ASCO Post. Available at: https://ascopost.com/issues/january-25-2023/camizestrant-improves-progression-free-survival-in-advanced-breast-cancer/
- www.ClinicalTrials.gov. (2021). A Comparative Study of AZD9833 Plus Palbociclib Versus Anastrozole Plus Palbociclib in Patients With ER-Positive HER2 Negative Breast Cancer Who Have Not Received Any Systemic Treatment for Advanced Disease. clinicaltrials.gov. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT04711252
- www.ClinicalTrials.gov. (2021). Phase III Study to Assess AZD9833+ CDK4/6 Inhibitor in HR+/HER2-MBC With Detectable ESR1m Before Progression (SERENA-6). clinicaltrials.gov. Available at: https://classic.clinicaltrials.gov/ct2/show/NCT04964934?term=Serena-6&draw=2&rank=1
- AstraZeneca. Datopotamab deruxtecan demonstrated statistically significant and clinically meaningful progression-free survival benefit in patients with HR-positive, HER2-low or negative breast cancer in TROPION-Breast01 Phase III trial. AstraZeneca. 2023. https://www.astrazeneca.com/media-centre/press-releases/2023/dato-dxd-improved-pfs-in-breast-cancer.html
- www.nice.org.uk. (n.d.). Elacestrant for treating postmenopausal hormone receptor-positive HER2-negative advanced or metastatic breast cancer after 1 or 2 endocrine treatments
[online] Available at: https://www.nice.org.uk/guidance/TA11263 [Accessed 16 January 2024]
