
In this blog we discuss how two different treatments for hormone positive breast cancer (tamoxifen vs. aromatase inhibitors) work.
Hormone Receptor Positive Breast Cancer
About 75% of breast cancers are positive for the oestrogen receptor (ER) and/or progesterone receptor (PR)1,2,3. Cases of hormone receptor (HR+) breast cancer increased between the years of 1990 and 2015, specifically in women aged 40 to 69 years. Although the reasons for this are not fully known, increased exposure to oestrogen caused by an earlier age of period onset, higher body mass index and increased use of hormone therapy (such as hormone replacement therapy in postmenopausal women) may contribute.
What are oestrogen and progesterone?
Oestrogen and progesterone are both hormones that travel in the blood to tell other parts of the body how to function. In women, oestrogen and progesterone are the vital sex hormones that regulate menstrual cycles and play an important role in pregnancy.
In breast cancer, these hormones have a very different function. Once they have made contact with cancer cells, they stimulate them to grow. This discovery has led to a whole new category of treatment called hormone therapy. Carry on reading to find out how this works!
What do these hormones have to do with breast cancer?
Of these hormones, oestrogen is the main female hormone stimulating breast cancer growth. ER-positive cancer cells have oestrogen receptors on the cell surface, and when these receptors are activated by oestradiol (a type of circulating oestrogen hormone), cells multiply and therefore the cancer grows4. Both tamoxifen and aromatase inhibitors (AIs) are hormonal therapies used in the treatment of oestrogen positive (ER-positive) breast cancers to stop tumour growth and recurrence.
Where is oestrogen produced?
In premenopausal women, oestrogen is mainly produced by the ovaries. However, for everyone including men, a small amount of oestrogen is also produced in other tissues, such as in fat cells, the breast, bone, liver and brain5. In these tissues, the enzyme aromatase is used to convert other hormones (such as testosterone) into oestradiol. After the menopause, there is no longer oestrogen production in the ovaries, but aromatase activity persists so oestrogen is still present.
How does Tamoxifen work?
Tamoxifen was the first specific hormone-based therapy to be used in breast cancer treatment that demonstrated significant clinical success6. This drug is used in the treatment of pre-menopausal and post-menopausal women, as well as men, whose breast cancer biopsies showed elevated levels of the oestrogen receptor. Tamoxifen works by blocking the oestrogen receptors on the cancer cells. This way, oestradiol is still present in normal amounts in the body, but it cannot activate the cancer cells to grow further (see diagram). 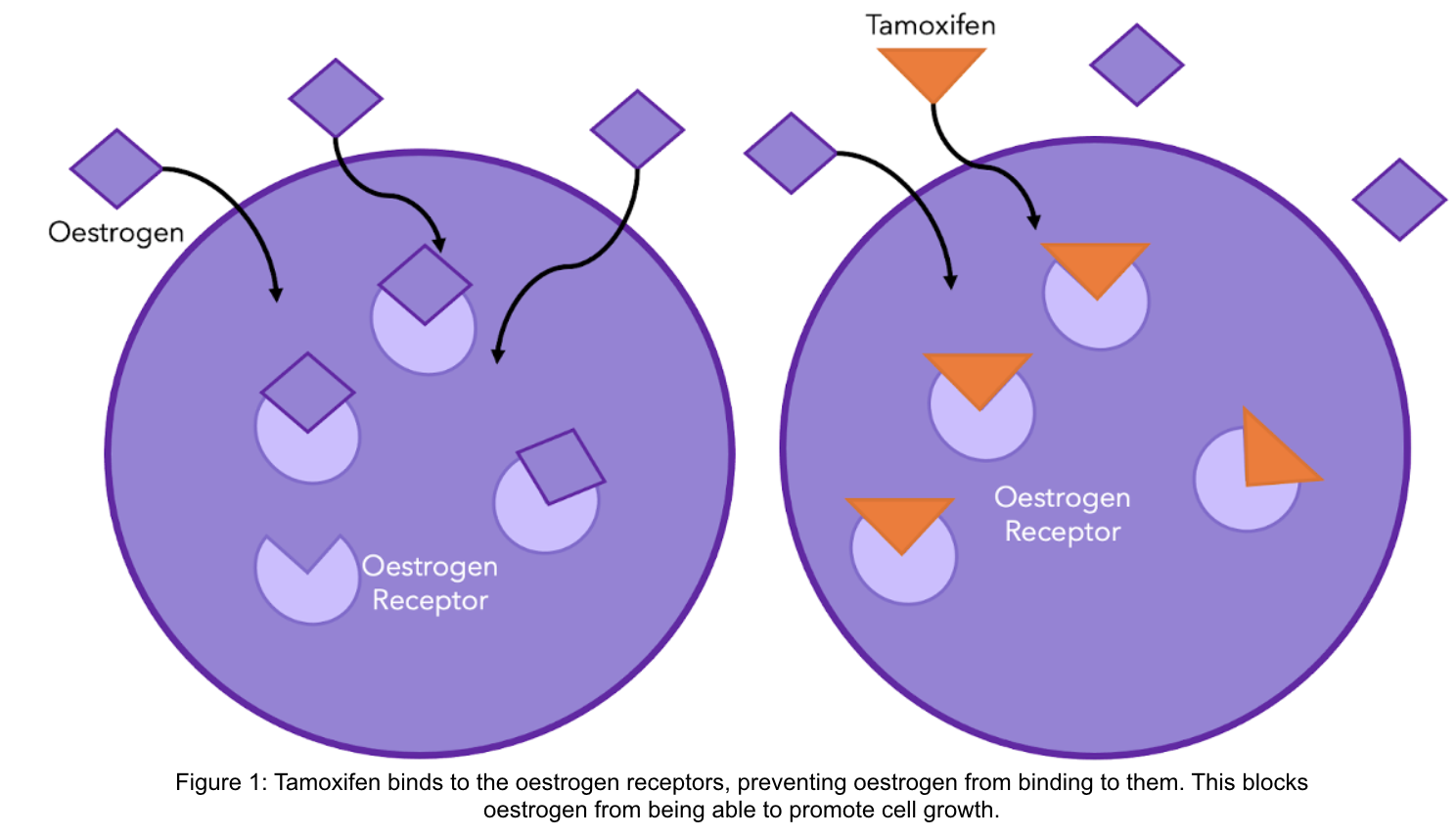
What happens when Tamoxifen doesn’t work?
However, some ER-positive patients do not respond to tamoxifen, or they may become resistant. Although the mechanisms to explain the resistance to tamoxifen remains unclear, one of the hypotheses is that when cells are “fed up” with this drug, they sometimes respond by elevating the expression of the gene “AKT”. This is a survival gene that helps to stimulate cell growth and proliferation in normal situations. In breast cancer, the AKT gene can become overactive and confer resistance by allowing cancer cells to continue using the oestrogen receptor even in the presence of tamoxifen7.
Another hypothesis why some patients might not benefit from tamoxifen is because of an enzyme called CYP2D6. This enzyme converts tamoxifen into its active form. In about 10% of people their CYP2D6 enzyme does not function well8. Having an abnormal CYP2D6 enzyme might prevent tamoxifen from blocking oestrogen receptors and cancer growth.
How do Aromatase Inhibitors work?
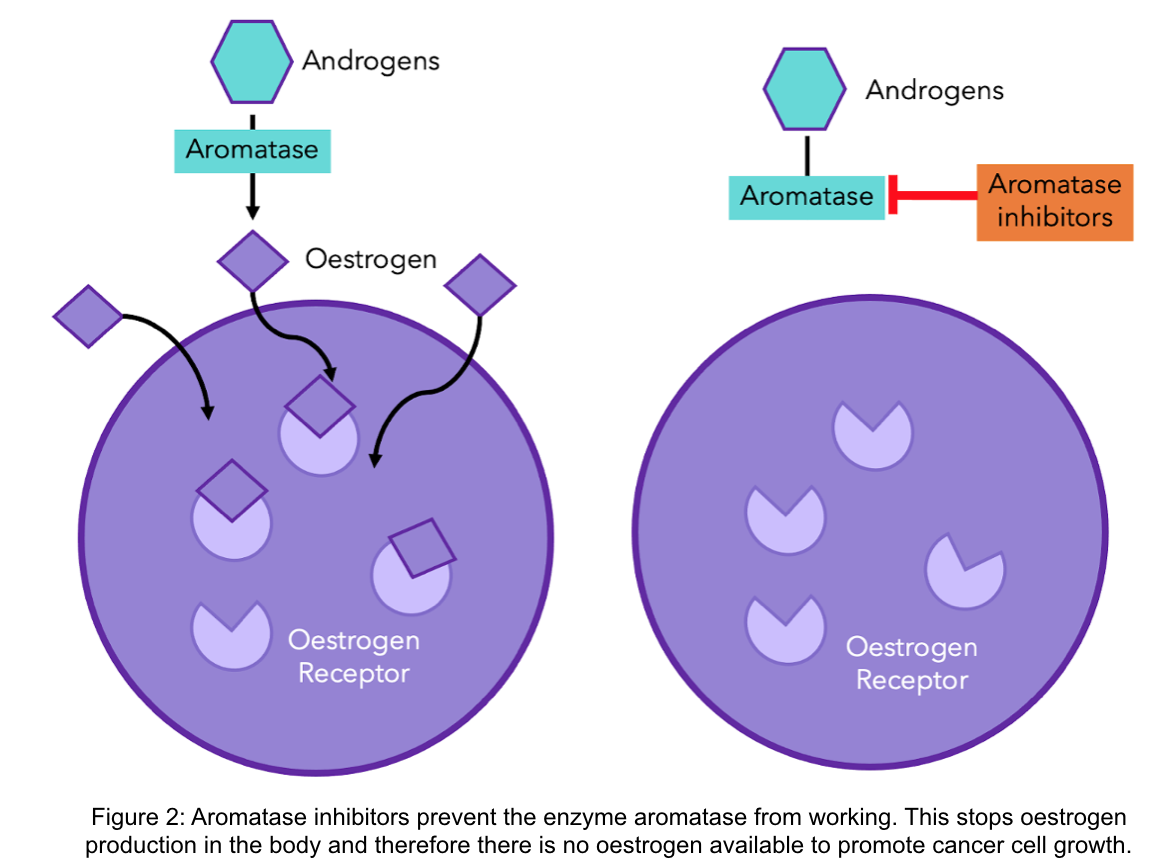
On the other hand, aromatase inhibitors (AIs), like anastrozole, letrozole or exemestane, are used to treat post-menopausal women and men with ER-positive breast cancer. AIs work by preventing the enzyme aromatase from doing its job of converting other hormones into oestradiol. Therefore, oestradiol production is stopped (see diagram). These inhibitors are much more effective than tamoxifen for post-menopausal women9,10.
Tamoxifen vs. Aromatase Inhibitors
Unlike tamoxifen, AIs are not often used to treat pre-menopausal breast cancer patients. This is because AIs cannot block the oestradiol production in the ovaries. However, a pre-menopausal woman can be treated with the aromatase inhibitor exemestane if their ovarian function is suppressed by drugs such as goserelin11. Letrozole, anastrozole and exemestane are third generation AIs and often decrease circulating oestrogen to undetectable levels12 thereby reducing tumour proliferation and growth13. Patients’ response rate to tamoxifen or aromatase inhibitor treatment can vary depending on their stage of life.
Pre-menopausal
A recent study compared four clinical trials that included 7030 pre-menopausal women with early-stage HR+ breast cancer receiving ovarian suppression14. Results showed that even though there was no difference between the two treatments in the number of patients still alive after 10 years of treatment (overall survival), there was a small difference in cancer recurrence between the two types of medication. The risk of the cancer returning in 5 years was 6.9% in patients on aromatase inhibitor treatment, in comparison to 10.1% receiving tamoxifen. A similar difference was found after 10 years of follow up: patients on aromatase inhibitors had a 14.7% risk of cancer recurrence, in comparison to 17.5% on tamoxifen treatment. However, data also showed that there were more bone fractures in patients on aromatase inhibitors (6.4%) compared to patients on tamoxifen (5.1%). Moreover, the occurrence of endometrial cancer was rare in aromatase inhibitor (0.2%) and tamoxifen (0.3%) patient groups.
Peri-menopausal
A study demonstrated that women aged 45-55 in their menopausal transitional phase (peri-menopause) with HR+ breast cancer experienced a benefit from aromatase inhibitor treatment15. A study including 304 peri-menopausal women showed 90% of patients being treated with aromatase inhibitors did not experience the cancer returning after 5 years, in comparison to 78% on tamoxifen treatment. There was also a benefit in terms of overall survival, as there was a 96% survival rate in patients taking aromatase inhibitors, compared to 87% in patients receiving tamoxifen.
Post-menopausal
In post-menopausal women with early HR+ breast cancer research suggests there is a significant benefit in being treated with aromatase inhibitors over tamoxifen. Data from 31,920 women were analysed and it was shown that patients on aromatase inhibitors had a 30% lower rate of the cancer coming back compared to patients on tamoxifen16. This study also showed that after 10 years 15% more patients were alive on aromatase inhibitors vs. tamoxifen. It should also be noted that there were fewer cases of endometrial cancer over 10 years in the aromatase inhibitor group (0.4%) compared to the tamoxifen group (1.2%). However, bone fractures were more common over 5 years in patients on aromatase inhibitors (8.2%) compared to patients on tamoxifen (5.5%). Another paper that compared four studies of approximately 2300 patients with secondary breast cancer similarly showed the benefit of aromatase inhibitors17. Those on aromatase inhibitors went on for longer without their cancer growing or worsening, in comparison to those on tamoxifen.
Keep track of your hormonal therapy side effects with OWise
 Hormonal therapies can trigger multiple side effects. Some of the most common include bone and joint pain, hot flashes, nausea, fatigue, mood swings, headaches, constipation, dry skin and loss of libido. With OWise, you can track them over time, and share your well-being data with yourself, doctors and loved ones.
Hormonal therapies can trigger multiple side effects. Some of the most common include bone and joint pain, hot flashes, nausea, fatigue, mood swings, headaches, constipation, dry skin and loss of libido. With OWise, you can track them over time, and share your well-being data with yourself, doctors and loved ones.
We hope this blog helped clarify the differences between tamoxifen vs. aromatase inhibitors. Try out the side-effects tracking and let us know if this article has helped you. Do you have any comments or suggestions? Contact us
References
- Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005; 123:21–27.
- Lange CA, Yee D. Progesterone and breast cancer. Womens Health (Lond) 2008; 4:151–162.
- Moelans CB, de Ligt J, van der Groep P, Prins P, Besselink N, Hoogstraat M, ter Hoeve N, Lacle M, Kornegoor R, van der Pol C, de Leng W, Barbe E, van der Vegt B, Martens J, Bult P, Smits VT, Koudijs M, Nijman I, Voest E, Selenica P, Weigelt B, Reis-Filho J, van der Wall E, Cuppen E, van Diest PJ. The molecular genetic make-up of male breast cancer. Endocr Relat Cancer. 2019
- Johnston SRD and Dowsett M. Aromatase Inhibitors for breast cancer: Lessons from the laboratory. Nature Reviews, 2003; 3: 821-831
- Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, Martin R, Utsunomiya H, Thung S, Gurates B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacological Reviews. 2005; 57:359–383
- Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007; 72:7–25.
- Bostner J, Karlsson E, Pandiyan MJ, Skoog L, Fornancder T, Nordenskjold B, Stal O. Activation of Akt, mTOR, and the estrogen receptor as a signature to predict tamoxifen treatment benefit. Breast Cancer Res Treat. 2013;137(2):397-406.
- Goetz MP, Kamal A, Ames MM. Tamoxifen pharmacogenomics: the role of CYP2D6 as a predictor of drug response. Clin Pharmacol Ther. 2008; 83(1): 160-6.
- Geisler J, King N, Dowsett M, Ottestad L, Lundgren S, Walton P, Kormeset PO, Lonning PE. Influence of anastrozole (Arimidex), a selective, non-steroidal aromatase inhibitor, on in vivo aromatisation and plasma oestrogen levels in postmenopausal women with breast cancer. Br J Cancer. 1996; 74:1286–1291.
- Geisler J, King N, Anker G, Ornati G, Salle ED, Lonning PE, Dowsett M. In-vivo inhibition of aromatization by exemestane, a novel irreversible aromatase inhibitor, in post-menopausal breast cancer patients. Clinical Cancer Research. 1998; 4: 2089-2093.
- Francis PA, Regan MM, Fleming GF, Láng I, Ciruelos E, Bellet M, Bonnefoi HR, Climent MA, Da Prada GA, Burstein HJ, Martino S, Davidson NE, Geyer CE Jr, Walley BA, Coleman R, Kerbrat P, Buchholz S, Ingle JN, Winer EP, Rabaglio-Poretti M, Maibach R, Ruepp B, Giobbie-Hurder A, Price KN, Colleoni M, Viale G, Coates AS, Goldhirsch A, Gelber RD; SOFT Investigators; International Breast Cancer Study Group. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015 Jan 29; 372(5): 436-46.
- Geisler J, Haynes B, Anker G, Dowsett M, Lønning PE. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 2002, 20: 751-757.
- Miller WR, Jackson J. The therapeutic potential of aromatase inhibitors. Expert Opin Investig Drugs. 2003, 12: 1-12. 10.1517/13543784.12.1.1.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: a patient-level meta-analysis of 7030 women from four randomised trials. Lancet Oncol. 2022, 23: 382-392.
- Dackus GMHE, Jóźwiak K, Sonke GS, van der Wall E, van Diest PJ, Hauptmann M, Siesling S, Linn SC. Optimal adjuvant endocrine treatment of ER+/HER2+ breast cancer patients by age at diagnosis: A population-based cohort study. European Journal of Cancer. 2018; 90: 92-101.
-
Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. The Lancet. 2015; 386(10001): 1341-1352.
-
Robertson John FR, Paridaens RJ, Lichfield J, Bradbury I, Campbell C. Meta-analyses of phase 3 randomised controlled trials of third generation aromatase inhibitors versus tamoxifen as first-line endocrine therapy in postmenopausal women with hormone receptor-positive advanced breast cancer. European Journal of Cancer. 2021; 145: 19-28.
