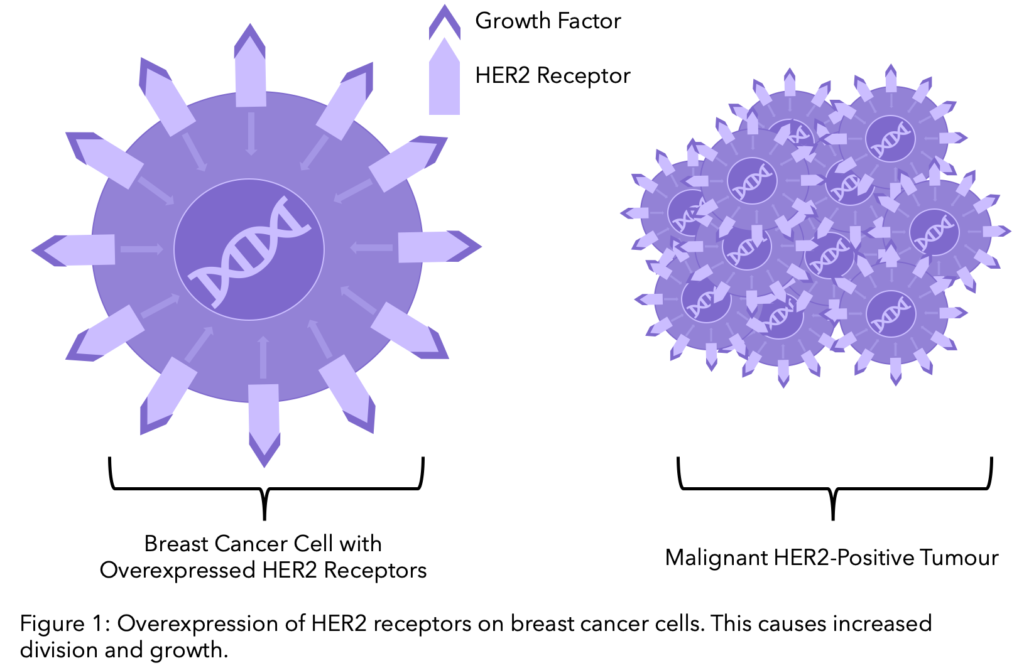
We’re kicking off our in-depth explorations of breast cancer subtypes with early HER2-positive breast cancer. 20-25% of breast cancers have high levels of HER2 protein1, which can influence outlook and treatment. Here we are going to explain exactly what HER2 is and how it can affect your treatment plan.
So, what is HER2?
Human Epidermal Growth Factor Receptor 2, commonly known as HER2, is a protein on the surface of cells and acts as a receptor, responding to signals from its environment. Normally HER2 is responsible for the healthy growth and development of cells. However, when the HER2 protein is present in high amounts (overexpressed) on the cell surface of breast cancer cells this leads to abnormal cell division and growth.
How do I know if I’m HER2-positive or negative?
The analysis of your tissue sample from a biopsy or surgery indicates if your tumour over expresses the HER2 protein. The expression of this protein is analysed by a staining technique called immunohistochemistry (IHC). If the IHC result is 0 or 1+, your sample is considered HER2-negative. If it is 3+ it is HER2-positive and if it is 2+ the result is inconclusive 2. You can review this in your pathology report. To find out more about the contents of your pathology report, head to our previous blog.
Are there treatments specific for HER2-positive breast cancer?
The significance of HER2 in the growth of breast cancer cells drove research for treatments that targeted the protein. This was first achieved by the organisation Genentech, who in the 1990’s developed Trastuzumab, commercially known as Herceptin®. Herceptin® is now the standard treatment for early and advanced HER2-positive breast cancer. However, the further discovery for treatments continued. Particularly in the last decade, we have seen a rise in the number of HER2 targeted therapies coming to the market.
Did you know?
The future of Herceptin® was almost halted by the disbanding of cancer drug development in Genentech. However in the late 1980’s, the mother of a senior Genentech vice president was diagnosed with breast cancer. “Just like that, one man flipped the switch on HER2.” He convinced his colleagues that Herceptin® was worth Genentech’s investment.
Robert Bazell, The Making of Herceptin®, a Revolutionary Treatment for Breast Cancer
Whilst this is invaluable for people to get the best care possible, with all the different terminology and decisions to be made, it can be easy to feel overwhelmed. So we have pulled together all the different HER2 treatment options you could have on the NHS. These are classified into three types: Monoclonal antibodies, antibody-drug conjugates and kinase inhibitors. In this blog, we are going to identify them all and explain how they work.
NICE is the British organisation that reviews new and existing drugs. In Scotland, NICE guidelines are not applied. They have the Scottish Medicines Consortium (SMC) and Scottish Intercollegiate Guidelines Network (SIGN) which approve new drugs and create guidelines, respectively. Therefore, the drugs available in NHS Scotland may differ to those in England 3.
Did you know you can add all your treatments into OWise, creating one easy-to-view treatment plan? The app also includes over 30 side effects and symptoms to choose from. You can track any changes and share these with your care team and loved ones. Better communication with your care team can make sure you receive the best care possible.
1. Monoclonal antibodies
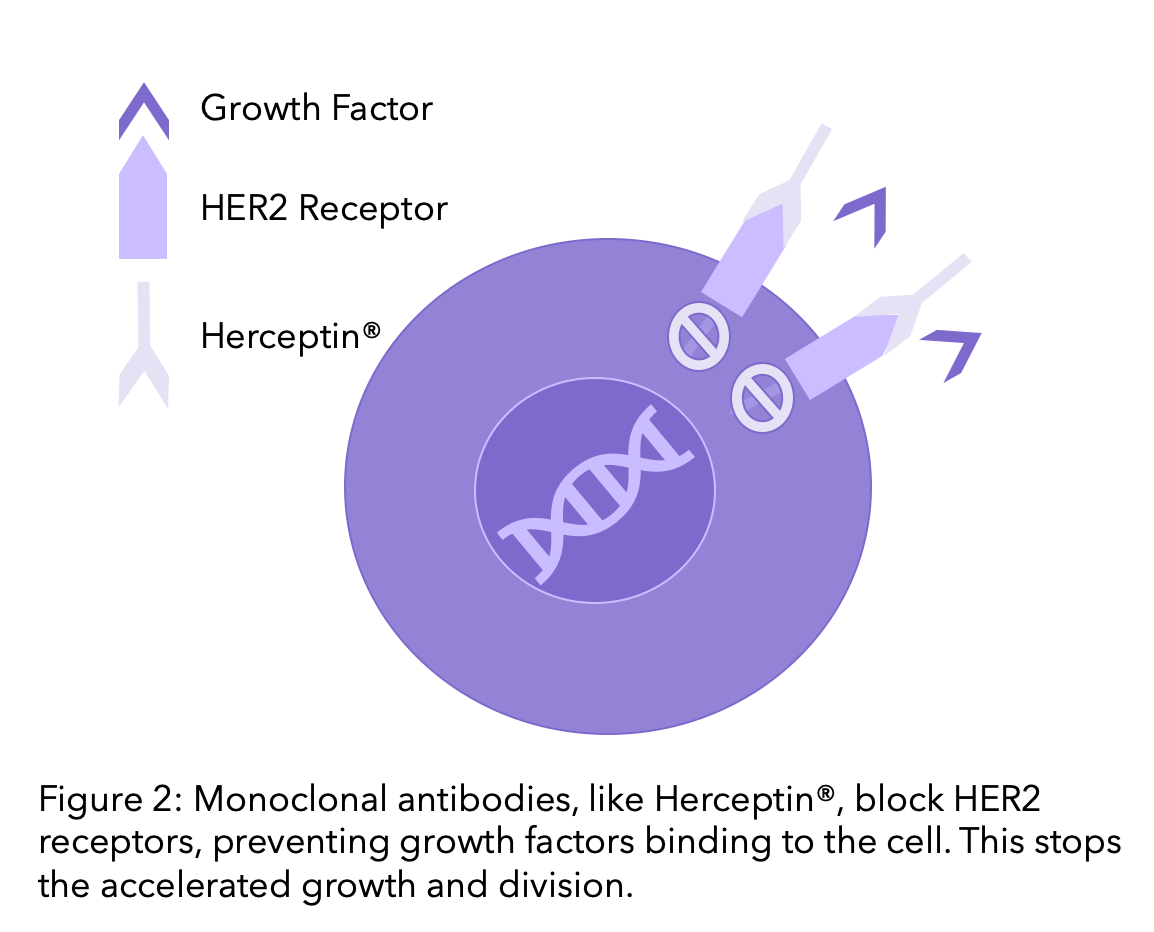
Monoclonal antibodies are drugs that block the HER2 receptor, blocking the pathway of HER2 and reducing the cell growth. To find out more on Monoclonal Antibodies, head to our previous blog. Trastuzumab and Pertuzumab are the most common monoclonal antibody therapies, which we will explore further here.
1.1 Trastuzumab (Herceptin®)
This first targeted therapy for HER2 is given intravenously for both early and advanced breast cancer, often alongside chemotherapy before or after surgery.
Herceptin® was the original brand name for the drug, however several similar versions (called biosimilars) are now available. Biosimilars for Herceptin® include Ogivri®, Herzuma®, Ontruzant®, Trazimera®, and Kanjinti®. They are clinically identical, with no changes in effectiveness. However, having multiple options on the drug market allows for the driving down of costs. Although practically identical in their treatment against cancer, they should not be used interchangeably and therefore you are commonly prescribed the brand name rather than the generic name6.
Did you know?
Herceptin® was a breakthrough for HER2-positive breast cancer subtypes. Clinical trials showed that the introduction of Herceptin® halved cancer recurrence and reduced mortality by 30%7,8,9. To find out more about the history of Herceptin®, check out our blog.
1.2 Pertuzumab (Perjeta®)
Pertuzumab, another HER2-specific monoclonal antibody, can be used for both primary and secondary breast cancer. It is included in standard care for neoadjuvant (before surgery) treatment in early breast cancer, alongside trastuzumab and chemotherapy. This is offered to people with locally advanced, inflammatory or early-stage breast cancer at high risk of recurrence12.
In the study that supported NICE’s decision to give pertuzumab as adjuvant (after surgery) therapy for early breast cancer, it was found that adding pertuzumab to standard trastuzumab and chemotherapy increased disease-free survival13. This means that after treatment, there was a decreased likelihood of breast cancer spreading to other parts of the body. This did not include the appearance of other types of cancer. This is offered to people with lymph node positive early breast cancer (N1 and higher in TNM staging), as the cancer is more likely to recur in this group.
Pertuzumab is also offered alongside trastuzumab and docetaxel for locally recurrent breast cancer in adults who have not had previous anti-HER2 therapy or chemotherapy and are unable to have surgery to remove the cancer. This was after a review in 2018 that found pertuzumab significantly improved progression-free and overall survival14.
1.3 Trastuzumab-hyaluronidase (PHESGO®) injection
In April 2021, an injection called PHESGO®️ was offered by the NHS for people with HER2 positive breast cancer. PHESGO®️ contains both pertuzumab with trastuzumab which were previously given as separate IV infusions. It also includes hyaluronidase protein which helps metabolise these targeted drugs so they can be administered together in one 5 min injection under the skin on your thigh. This significantly cuts treatment time.
Whether administration via injection or IV infusion would be suitable depends on whether the patient can receive treatments at home, the local support that is available and whether other IV infused treatments are included in their treatment plan. If they are receiving other IV treatments, having the injection at home would not save a significant time in hospital, one of the main benefits of the PHESGO injection11.
2. Antibody drug conjugates
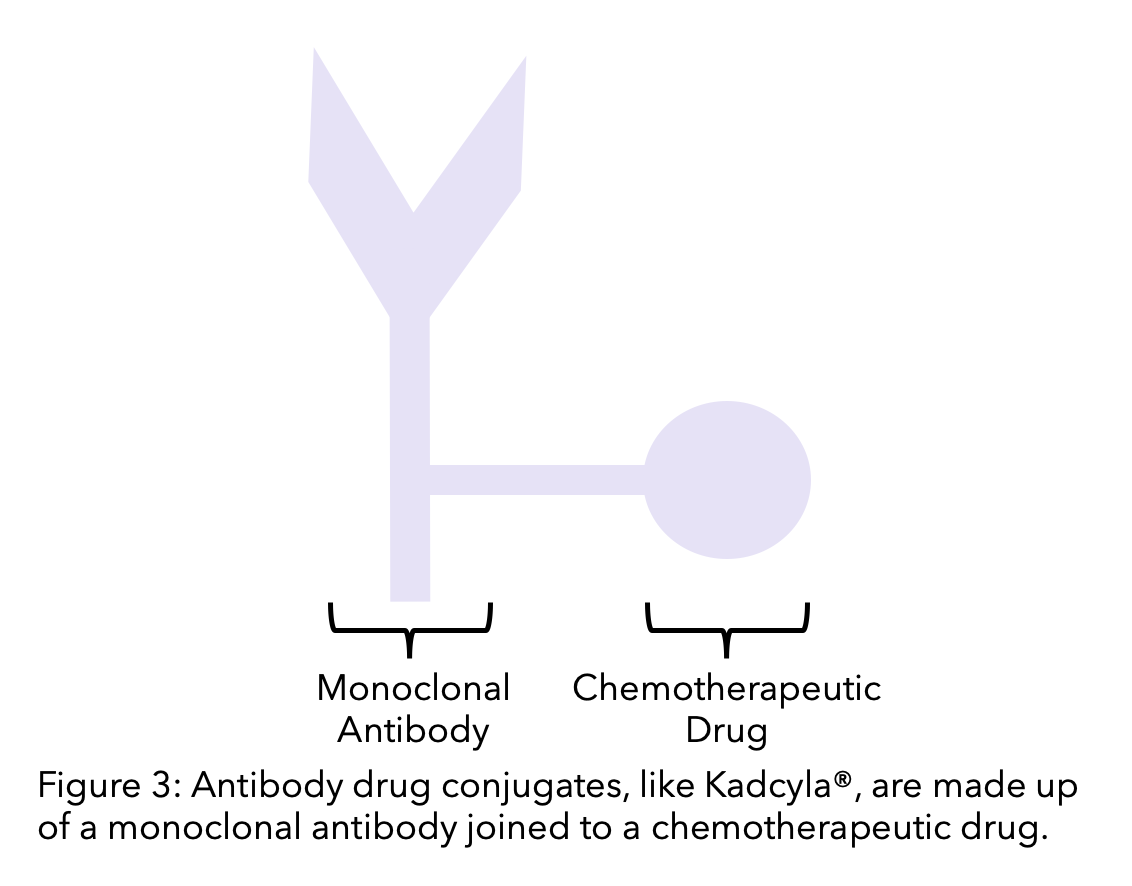
An antibody-drug conjugate (ADC) is a monoclonal antibody linked to a chemotherapy drug. The monoclonal antibody targets and binds to the cell. The chemotherapeutic agent then becomes active when it enters the cell. This means that HER2-positive breast cancer cells can be targeted, and the cytotoxic effects of chemotherapy concentrated to the cancer cells.
2.1 Ado-trastuzumab emtansine (Kadcyla® or TDM-1)
Kadcyla® is an intravenous drug made up of trastuzumab and the chemotherapy drug emtansine. Kadcyla® is approved by NICE to be given for early breast cancer in the NHS. Clinical trial evidence showed that the use of Kadcyla increases the time people remain free of disease compared to trastuzumab alone16.
“Additional treatment options that can increase the amount of time in which people remain free of disease after surgery, and perhaps stop it from coming back altogether, are particularly welcome. We are therefore pleased to be able to recommend that trastuzumab emtansine is made available routinely for people with HER2-positive early breast cancer after surgery.”
Meindert Boysen, deputy chief executive and director of the Centre for Health Technology Evaluation at NICE
Kadcyla® can be recommended to people with early HER2+ breast cancer who still have cancer remaining after receiving treatment before surgery. Trastuzumab emtansine can also be a treatment option for HER2+ breast cancer that is locally recurrent or cannot be operated on and has been previously treated with trastuzumab and taxane chemotherapy. The patient must have been previously treated for locally advanced cancer or developed a recurrence within 6 months of finishing treatment after surgery17.
2.2 Trastuzumab deruxtecan (Enhertu®)
You may have heard the hype around Enhertu®, or trastuzumab deruxtecan, over the past few years. Enhertu is an innovative targeted therapy that can be used to treat some types of metastatic breast cancers. Much of the excitement surrounding Enhertu stems from extremely promising trial results that have even been described as ‘unprecedented’. Many experts believe that Enhertu will dramatically change how breast cancer is treated, not just for HER2-positive cases but also for many cancer previously considered HER2-negative.
Trastuzumab deruxtecan, brand name Enhertu®, is trastuzumab joined with the chemotherapy agent deruxtecan, administered intravenously. It is approved as a second line treatment option for people who have had previous treatment for secondary HER2+ breast cancer that was unsuccessful in the UK, EU and US30,31,32.
Enhertu was approved to treat HER2-low breast cancer patients in the US, EU and Scotland32,33,34. However, Enhertu was rejected by NICE for the treatment of HER2-low breast cancer, therefore is not available on the NHS in the UK. The NICE guidance will be reviewed if there is any evidence that is likely to change the recommendations.
HER2-low is a new way to classify breast cancer, it includes some hormone receptor-positive (HR+) and triple negative breast cancers (TNBC) that were previously considered HER2-negative (HER2-). Patients must have received prior chemotherapy to treat secondary breast cancer or developed a recurrence during or within six months of completing chemotherapy after surgery (adjuvant treatment) to be eligible for treatment33.
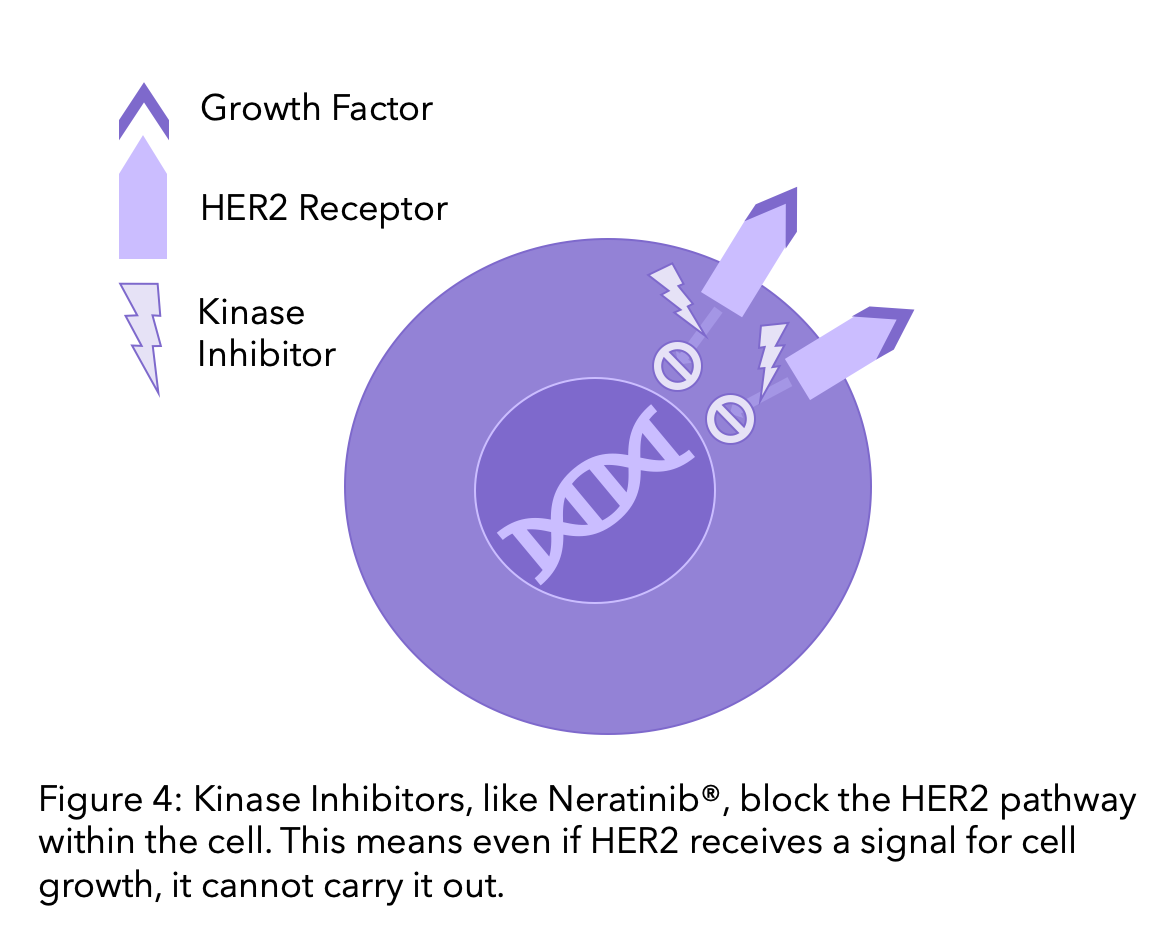
3. Kinase inhibitors
HER2 is a type of protein known as a kinase. Kinases are proteins in cells that receive signals to prompt the cell to grow, divide or die. Therapies that block kinases are called kinase inhibitors. Whilst monoclonal antibodies block the HER2 receptor by binding to it outside the cell, kinase inhibitors block the pathway within the cell. This means that if they receive a signal that the cell should divide or grow, the protein cannot carry it out.
3.1 Neratinib
Neratinib (Nerlynx®) are oral pills used in HER2+ and Hormone Receptor-positive, early-stage breast cancer. They can be given if there are still cancer cells remaining after neoadjuvant (before surgery) chemotherapy. The treatment is given after the adjuvant (after surgery) trastuzumab (sometimes called Herceptin®) treatment, which should have completed less than a year ago21.
What side effects will I be at risk of?
There are some common side effects for all HER2 therapies, however each drug varies in specific side effects that you may be at high risk of. There are some excellent resources online that give you a breakdown of the top side effects, such as Cancer Research UK’s A-Z list of cancer drugs.
It is well known that cardiac toxicity (heart dysfunction) is one of the most common adverse effects among patients receiving chemotherapy and HER2-directed therapy such as trastuzumab, pertuzumab or TDM-1. Therefore people receiving trastuzumab typically undergo cardiac monitoring, and treatment should be stopped if a heart measure called left ventricular ejection fraction (LEVF) drops by 10% from baseline and to below 50%25. However, what is less clear are the long-term cardiac complications for breast cancer survivors who received such therapy in the past26. Recent studies have suggested that exercise training such as high intensity training could help prevent cardiac toxicity after cancer treatment27.
Other common side effects you may experience are28:
- Sleep problems
- Muscle/joint pain
- Redness at the IV site
- Diarrhoea
- Nausea
- Tiredness
- Headache
- Mouth sores
- Loss of appetite
- Cold symptoms
- Rash
Neratinib and the combination of pertuzumab with trastuzumab can cause severe diarrhoea, so it is crucial to let your health team know any changes in bowel habits as they happen.
Some kinase inhibitors, including neratinib, can cause liver problems. Regular blood tests will be taken to check your liver function, but watch out for itchy or yellowing of the skin, dark urine or pain in the right upper belly area29.
And that’s HER2-positive treatment summed up
We hope that you now better understand your treatment options and can feel confident in discussions with your care team. Whilst we have included early HER2-positive treatment currently available on the NHS, there are currently clinical trials being run and new treatments may become available in the near future. We want to make sure you are kept informed so make sure to follow our Instagram and Twitter for any updates.
References
- Arteaga, C., Sliwkowski, M., Osborne, K., Perez, E., Puglisi, F., Gianni, L. 2011. Treatment of HER2-positive breast cancer: current status and future perspectives. Nat. Rev. Clin. Oncol. 9, 16–32.
- Cancer.org. 2020. Breast Cancer HER2 Status | HER2-positive Breast Cancer. [online] Available at: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-her2-status.html [Accessed 7 October 2020].
- Cancerresearchuk.org. 2019. Scotish Medicine Consortium (SMC). [online] Available at: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/access-to-treatment/scottish-medicines-consortium-smc [Accessed 7 October 2020].
- Nice.org.uk. 2020. NHS England interim treatment options during theCOVID-19 pandemic [online]. Available at: https://www.nice.org.uk/guidance/ng161/resources/interim-treatment-change-options-during-the-covid19-pandemic-endorsed-by-nhs-england-pdf-8715724381
- Nice.org.uk. 2020. Overview | Guidance On The Use Of Trastuzumab For The Treatment Of Advanced Breast Cancer | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/TA34 [Accessed 6 October 2020].
- England.nhs.uk. 2020. What Is A Biosimilar Medicine?. [online] Available at: https://www.england.nhs.uk/wp-content/uploads/2019/05/what-is-a-biosimilar-medicine-guide-v2.pdf [Accessed 6 October 2020].
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354(8):809‐820.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673‐1684.
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659‐1672.
- Ismael, G., Hegg, R., Muehlbauer, S., Heinzmann, D., Lum, B., Kim, S., Pienkowski, T., Lichinitser, M., Semiglazov, V., Melichar, B. and Jackisch, C., 2012. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I–III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. The Lancet Oncology, 13(9), pp.869-878.
- Duco, M., Murdock, J. and Reeves, D., 2019. Trastuzumab/Hyaluronidase-oysk: A New Option for Patients With HER2-Positive Breast Cancer. Annals of Pharmacotherapy, 54(3), pp.254-261.
- Nice.org.uk. 2020. 1 Recommendations | Pertuzumab For Adjuvant Treatment Of HER2-Positive Early Stage Breast Cancer | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/ta569/chapter/1-Recommendations [Accessed 6 October 2020].
- Von Minckwitz,G., Procter, M., de Azambuja, E., Zardavas,D., Benyunes, M., Viale, G., Suter, T., Arahmani, A., Rouchet N., Clark, E., Knott, A., Lang, I.,Levy, C., Yardley, C., Bines, J., Gelber, R., Piccart, M., Baselga, J., 2017. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. New England Journal of Medicine. 377(7), pp.702-702.
- Nice.org.uk. 2018. Pertuzumab with trastuzumab and docetaxel for treating HER2-positive breast cancer | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/ta509 [Accessed 6 October 2020].
- breastcancernow.org. 2019. Breast Cancer Now responds to the Scottish Medicine Consortium’s decisions on breast cancer drugs Perjeta and Decapeptyl SR. [online]. Available at: https://breastcancernow.org/about-us/media/press-releases/breast-cancer-now-responds-scottish-medicine-consortium’s-decisions-breast-cancer-drugs-perjeta-decapeptyl-sr [Accessed 7 October 2020].
- NICE. 2020. NICE Draft Guidance Recommends New Treatment Option For People With Early Breast Cancer. [online] Available at: https://www.nice.org.uk/news/article/nice-draft-guidance-recommends-new-treatment-option-for-people-with-early-breast-cancer [Accessed 6 October 2020].
- Nice.org.uk. 2016. Trastuzumab emtansine for treating HER2-positive advanced breast cancer after trastuzumab and a taxane | Guidance | NICE. [online] Available at: https://www.nice.org.uk/Guidance/TA458 [Accessed 6 October 2020].
- Nice.org.uk. 2020. Trastuzumab Deruxtecan For Treating HER2-Positive Unresectable Or Metastatic Breast Cancer After 2 Or More Anti-HER2 Therapies. [online] Available at: https://www.nice.org.uk/guidance/gid-ta10582/documents/final-scope [Accessed 6 October 2020].
- Nice.org.uk. 2020. Project Information | Trastuzumab Deruxtecan For Treating HER2-Positive Unresectable Or Metastatic Breast Cancer After 2 Or More Anti-HER2 Therapies [ID2697] | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/indevelopment/gid-ta10582 [Accessed 6 October 2020].
- Nice.org.uk. 2020. 1 Guidance | Lapatinib Or Trastuzumab In Combination With An Aromatase Inhibitor For The First-Line Treatment Of Metastatic Hormone-Receptor-Positive Breast Cancer That Overexpresses HER2 | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/ta257/chapter/1-Guidance [Accessed 6 October 2020].
- Nice.org.uk. 2020. 1 Recommendations | Neratinib For Extended Adjuvant Treatment Of Hormone Receptor-Positive, HER2-Positive Early Stage Breast Cancer After Adjuvant Trastuzumab | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/ta612/chapter/1-Recommendations [Accessed 6 October 2020].
- Nice.org.uk. 2020. Project Information | Neratinib For Treating HER2-Positive Breast Cancer After 2 Therapies [ID1381] | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/indevelopment/gid-ta10539 [Accessed 6 October 2020].
- Clinicaltrials.gov. 2020. A Study Of Tucatinib Vs. Placebo In Combination With Capecitabine & Trastuzumab In Patients With Advanced HER2+ Breast Cancer – Full Text View – Clinicaltrials.Gov. [online] Available at: https://clinicaltrials.gov/ct2/show/NCT02614794[Accessed 6 October 2020].
- National Cancer Institute. 2020. New Treatments Emerge For Metastatic HER2+ Breast Cancer. [online] Available at: https://www.cancer.gov/news-events/cancer-currents-blog/2020/tucatinib-trastuzumab-deruxtecan-her2-positive-metastatic-breast-cancer [Accessed 6 October 2020].
- Nice.org.uk. 2020. Trastuzumab for the adjuvant treatment of early-stage HER2-positive breast cancer. [online] Available at: https://www.nice.org.uk/guidance/ta107/documents/final-appraisal-determination-breast-cancer-early-trastuzumab2 [Accessed 7 October 2020].
- Huszno, J., Leś, D., Sarzyczny-Słota, D., Nowara, E., 2013. Cardiac side effects of trastuzumab in breast cancer patients – single centere experiences. Contemp Oncol. 2, 190-195.
- Scott, J. M., Nilsen, T. S., Gupta, D., & Jones, L. W., 2018. Exercise Therapy and Cardiovascular Toxicity in Cancer. Circulation, 137(11), 1176-1191.
- Konkel, L. 2020. Chemo and Targeted Therapy for HER2-Positive Breast Cancer. [online] Available at: https://www.healthline.com/health/breast-cancer/chemotherapy-for-her2-positive-breast-cancer [Accessed 6 October 2020].
- American Cancer Society. 2020. Targeted Therapy for Breast Cancer. [online] Available at: https://www.cancer.org/cancer/breast-cancer/treatment/targeted-therapy-for-breast-cancer.html [Accessed 6 October 2020].
- Trastuzumab deruxtecan for treating HER2-positive unresectable or metastatic breast cancer after 1 or more anti-HER2 treatments. 2023. Available at: https://www.nice.org.uk/guidance/ta862.
- FDA grants regular approval to fam-trastuzumab deruxtecan-nxki for breast cancer. 2022. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-fam-trastuzumab-deruxtecan-nxki-breast-cancer.
- Enhertu. 2021. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/enhertu
- FDA approves first targeted therapy for HER2-low breast cancer. 2022. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer
- Scottish Medicines Consortium. Trastuzumab deruxtecan (Enhertu). 2023. Available at: https://scottishmedicines.org.uk/medicines-advice/trastuzumab-deruxtecan-enhertu-full-smc2608/
- NICE. Trastuzumab deruxtecan for treating HER2-low metastatic or unresectable breast cancer after chemotherapy. 2024. Available at: https://www.nice.org.uk/guidance/TA992