
HER2-low is a new way to characterise breast cancer. You may be wondering what this means and how it may affect you. In short, many previously considered HER2-negative breast cancer patients can now be considered HER2-low. This provides a new treatment target for many secondary (metastatic, advanced) breast cancers. This blog will cover HER2-low by delving into what it is, how it is measured, how common it is, approved treatments and other research.
What is HER2?
Firstly, it’s important to understand what HER2 is. HER2, which is short for human epidermal growth factor receptor 2, is a protein that is responsible for the healthy growth and development of cells. In HER2-positive (HER2+) breast cancer patients, the HER2 protein is present in high amounts (overexpressed) on the cell surface of breast cancer cells. The overexpression of HER2 leads to abnormal cell division and growth of cancer cells.
You can read more about HER2+ breast cancer here.
How is HER2 status measured?
After a tumour biopsy sample has been taken, HER2 status is usually measured by an immunohistochemistry (IHC) test, which measures how much HER2 is present on the cell surface. This is often followed by another test that looks at the DNA of cancer cells to see if there are high levels of the HER2 gene present; this is called a fluorescence in situ hybridisation (FISH) test. IHC results range from 0-3+, whereas FISH results are either positive or negative.
Table 1. HER2 Status: HER2-negative, HER2-low, and HER2-positive breast cancer defined by immunohistochemistry and fluorescence in situ hybridisation test results.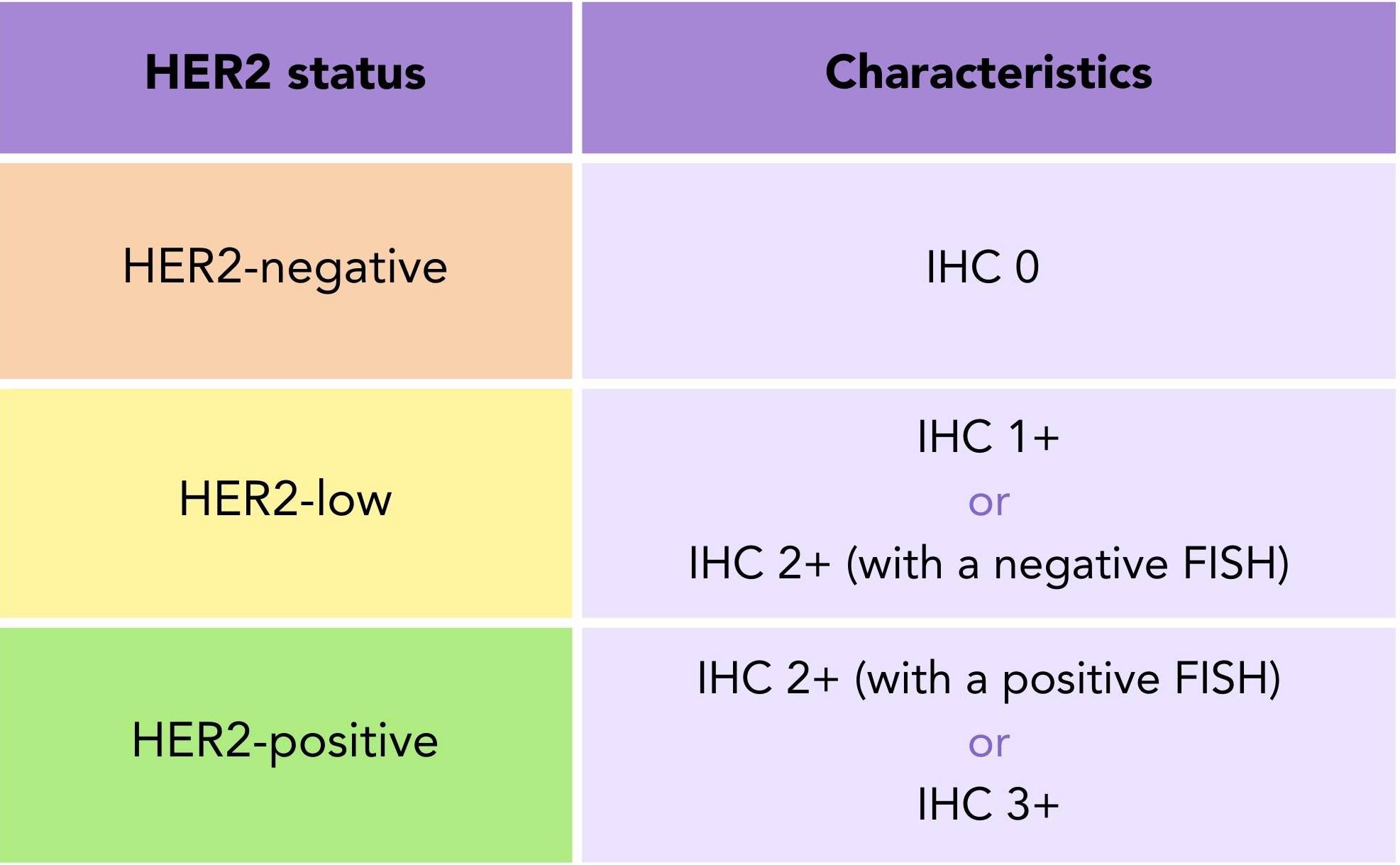
A sample is considered HER2-negative (HER2-) with an IHC score of 0, HER2-low with an IHC score of 1+ or an IHC score of 2+ with a negative FISH result, and HER2+ with an IHC score of 2+ with a positive fish or an IHC score of 3+ (table 1).
How common is HER2-low?
Research suggests that with the newly defined HER2 characteristics, up to 55% of breast cancers are now considered HER2-low1 (figure 1) .
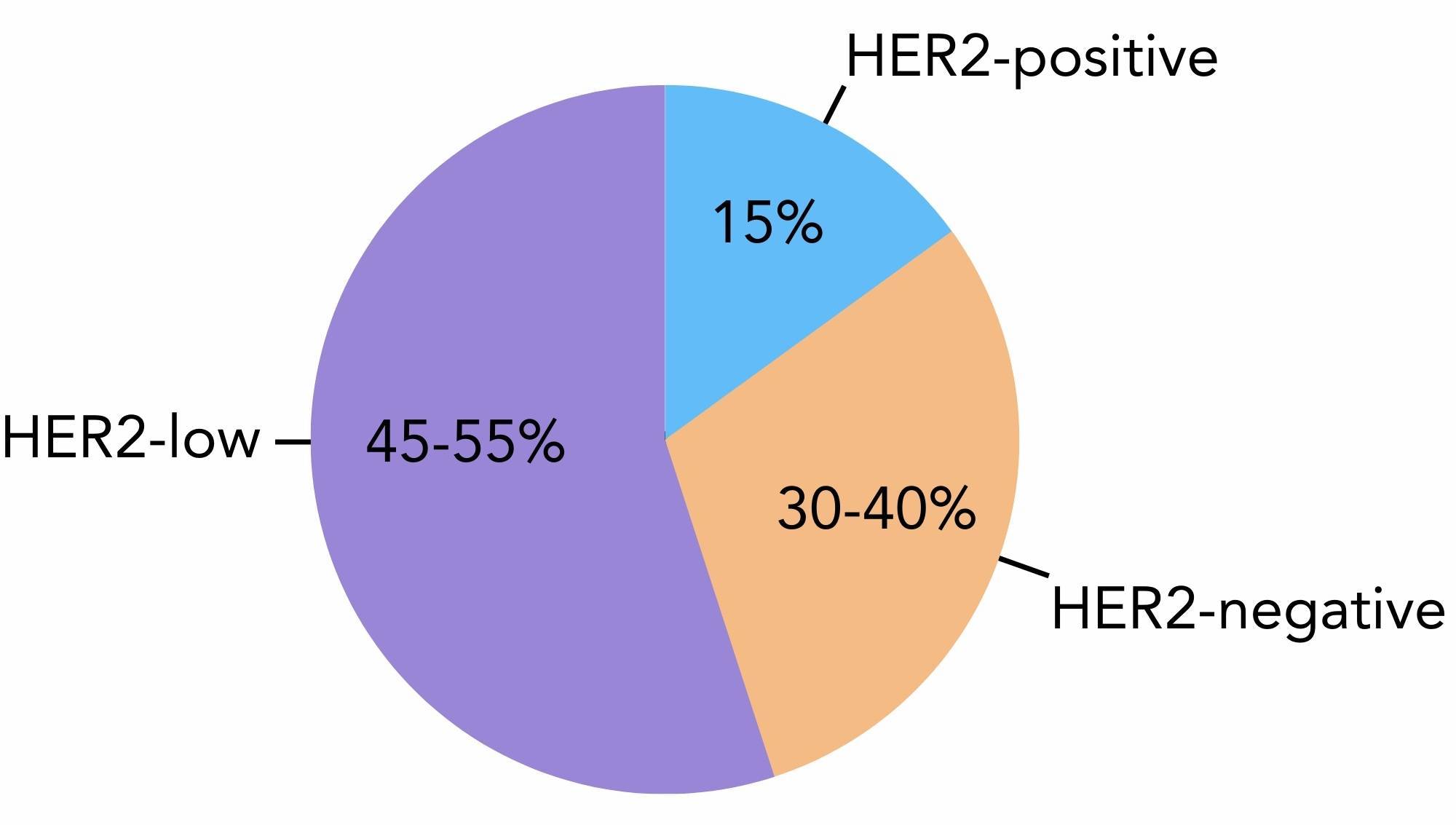
Figure 1. The amount of breast cancer cases that are considered HER2-positive (15%), HER2-low (45-55%) and HER2-negative (30-40%).
It has been found that HER2-low is more common in the hormone receptor-positive (HR+) subtype than triple negative breast cancers (TNBC). Results from a study published in 2023 found that of 1464 previously considered HER2- cases, 412 were HER2-low. Data showed the proportion of breast cancer subtypes in the HER2-low population1:
- Oestrogen receptor-positive (ER+): 83%
- Progesterone receptor-positive (PR+): 75.9%
- TNBC: 17-24.1%
What is the impact of HER2-low?
Until recently, samples were either considered HER2- or HER2+. This excluded the HER2- patients from being eligible to receive any HER2 targeted treatments.
If you were previously classified as HER2-, it is possible that you could now fall into the HER2-low category. This new development has significant implications for the treatment landscape of secondary HR+ and TNBC patients who are now HER2-low. The use of HER2 targeted therapies may provide a further treatment option for these patients after being treated with one previous line of chemotherapy.
“The takeaway from all this is that we have many new options. We have this new problem deciding what to use. We’ve never had this. All these choices are good to have because you can adapt treatment.” Paolo Tarantino, MD2
If you want to know your HER2 status you can check your pathology report or ask your care team what your status is.
Do you know that you can create question lists on OWise? You can choose from a list of suggested questions or add your own. Keep track of the answers and important information through adding text, photos or recordings. Asking questions can lead to better communication with your care team can ensure you receive the best care possible.
Are any treatments approved to treat HER2-low breast cancer?
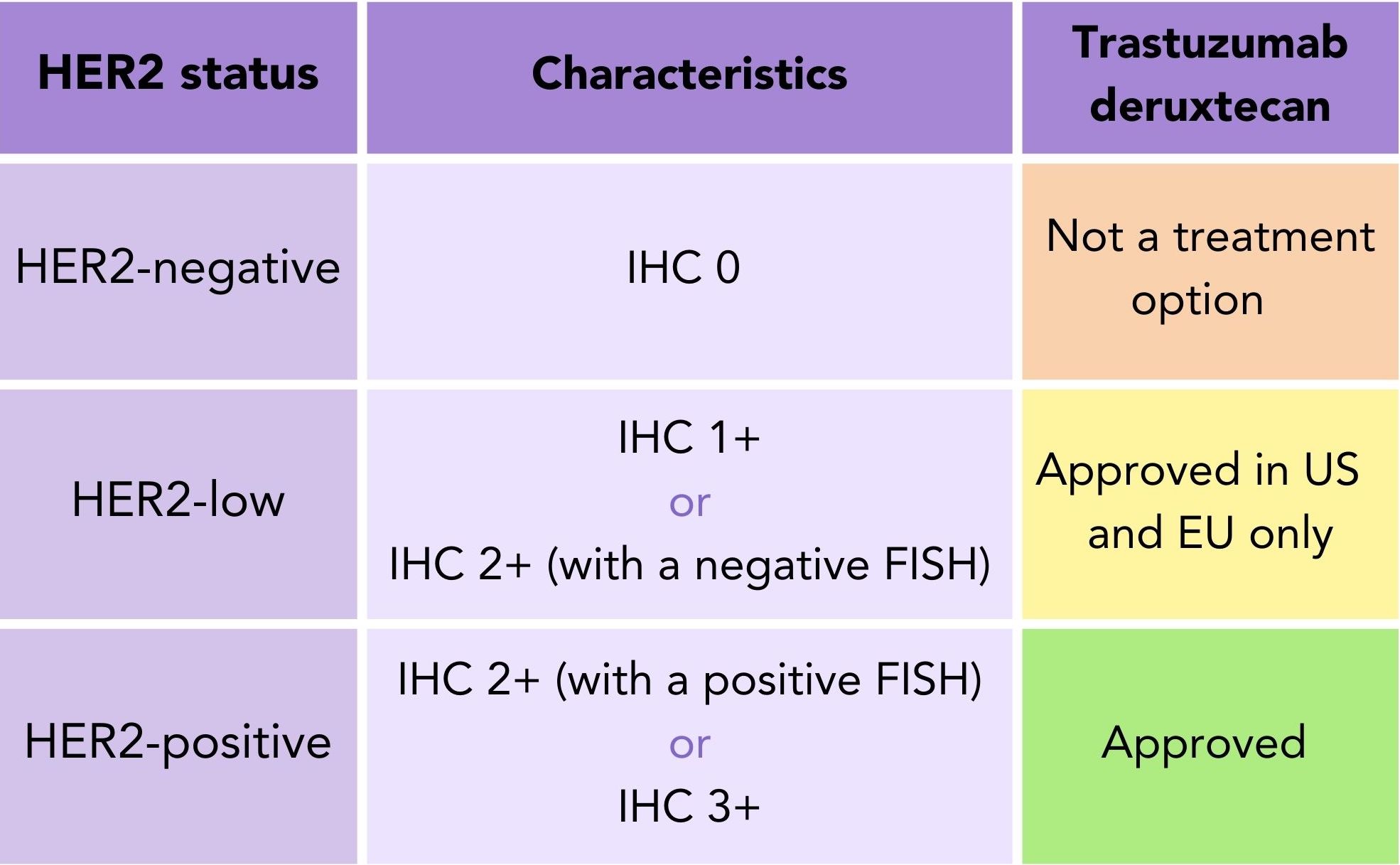
Trastuzumab deruxtecan was recently approved to treat secondary HER2-low breast cancer patients in the US and EU3,4 (table 2). Trastuzumab deruxtecan is currently under review by NICE in the UK as a treatment for HER2-low secondary breast cancer and a decision is expected in November 20235.
Table 2. HER2 Status: Trastuzumab deruxtecan approval to treat secondary breast cancer based on HER2 status (HER2-negative, HER2-low and HER2-positive).
What is trastuzumab deruxtecan (Enhertu®)?
Trastuzumab deruxtecan (Enhertu®) is a type of targeted therapy called an antibody-drug conjugate (ADC). ADCs consist of a monoclonal antibody and chemotherapy drug joined together by a chemical linker6.
What are antibody-drug conjugates?
Monoclonal antibodies (also called mAbs) are proteins that are made in the lab that can bind to specific targets (antigens) on the surface of cells7. Chemotherapy (often called chemo) involves using anti-cancer drugs to interfere how cancer cells grow and divide. Chemotherapy targets fast-dividing cells non-specifically, meaning it can destroy healthy cells as well as cancer cells8. ADCs are designed to target antigens present on cancer cells and deliver the chemotherapy drug directly to the site of tumour cells, reducing the impact of chemotherapy on healthy cells thereby leading to fewer side effects6,9. In this case, trastuzumab deruxtecan consists of the monoclonal antibody trastuzumab linked to the chemotherapy drug deruxtecan (figure 2).
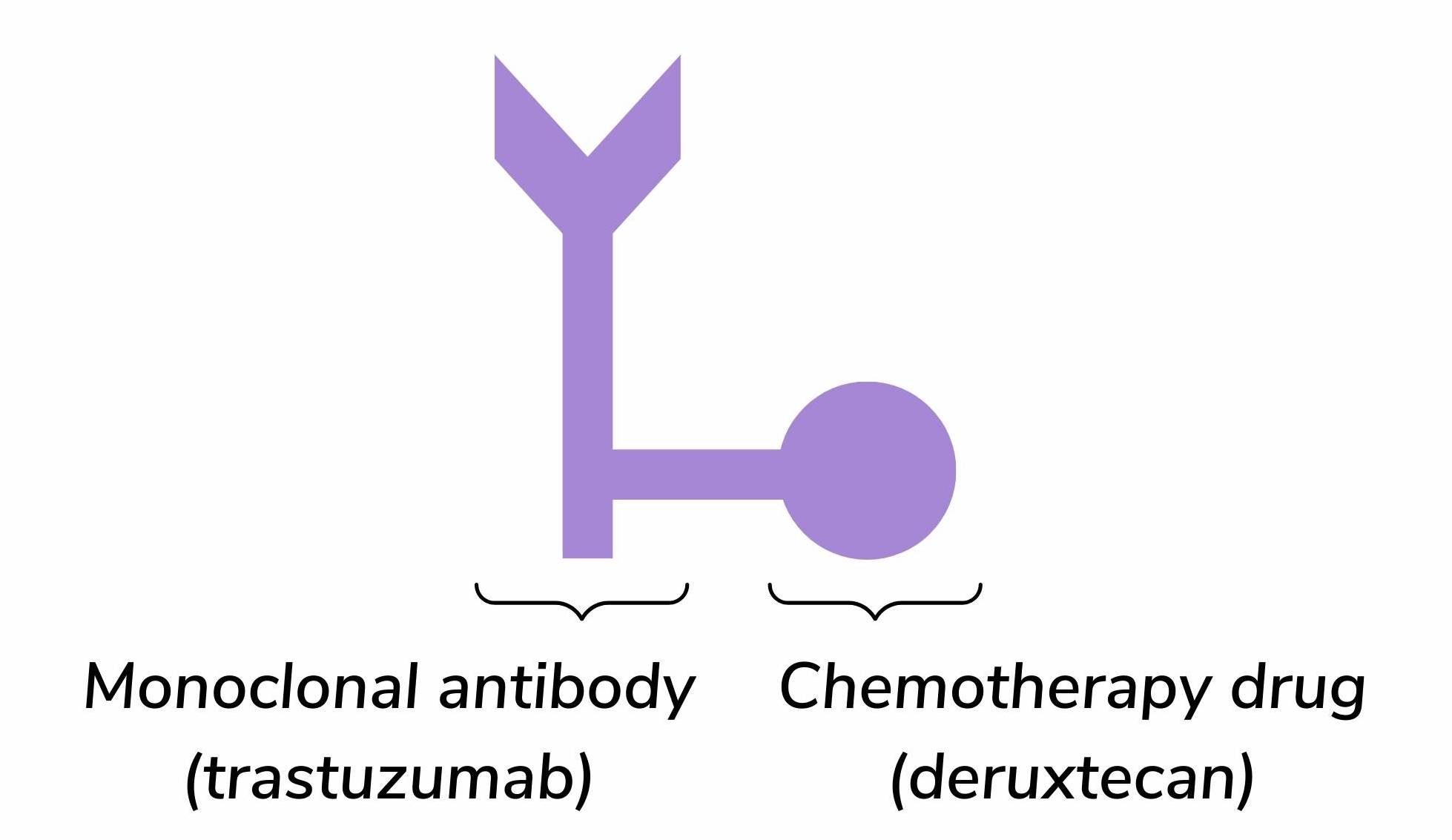
Figure 2. The structure of trastuzumab deruxtecan (Enhertu®).
How does trastuzumab deruxtecan (Enhertu®) work?
The trastuzumab monoclonal antibody part specifically targets and binds to HER2 proteins that are present in high amounts of the surface of cancer cells. When trastuzumab binds to HER2 the chemical linker is split by enzymes at the site of the cancer cells10. This leads to the deruxtecan drug being released and taken in by the cancer cells. Deruxtecan prevents cancer cells from growing and dividing, resulting in cell death10,11. This highly targeted mechanism is designed to minimise damage to healthy cells, improve the impact of the drug and reduce side effects6.
To be eligible for treatment, patients must have received prior chemotherapy to treat secondary breast cancer or developed a recurrence during or within six months of completing chemotherapy after surgery (adjuvant treatment).
Clinical data for trastuzumab deruxtecan (Enhertu®)
How long did HER2-low patients go without the cancer growing or spreading on trastuzumab deruxtecan (Enhertu®)?
The DESTINY-Breast04 trial looked at trastuzumab deruxtecan in the HER2-low population and compared trastuzumab deruxtecan with chemotherapy. This trial consisted of 540 patients, 480 of these patients had hormone receptor-positive (HR+) breast cancer and 60 had triple negative breast cancer (TNBC). The results showed that for patients on trastuzumab deruxtecan the cancer did not grow or spread for 9.9 months while, compared to 5.1 months for those on chemotherapy12.
These results varied depending on the subtype of breast cancer. For HR+ patients, most went on without the cancer growing or spreading for 10.1 months on trastuzumab deruxtecan, compared to 5.4 months on chemotherapy (figure 3). For TNBC patients these figures were 8.5 months on trastuzumab deruxtecan, compared to 2.9 months on chemotherapy12 (figure 3).
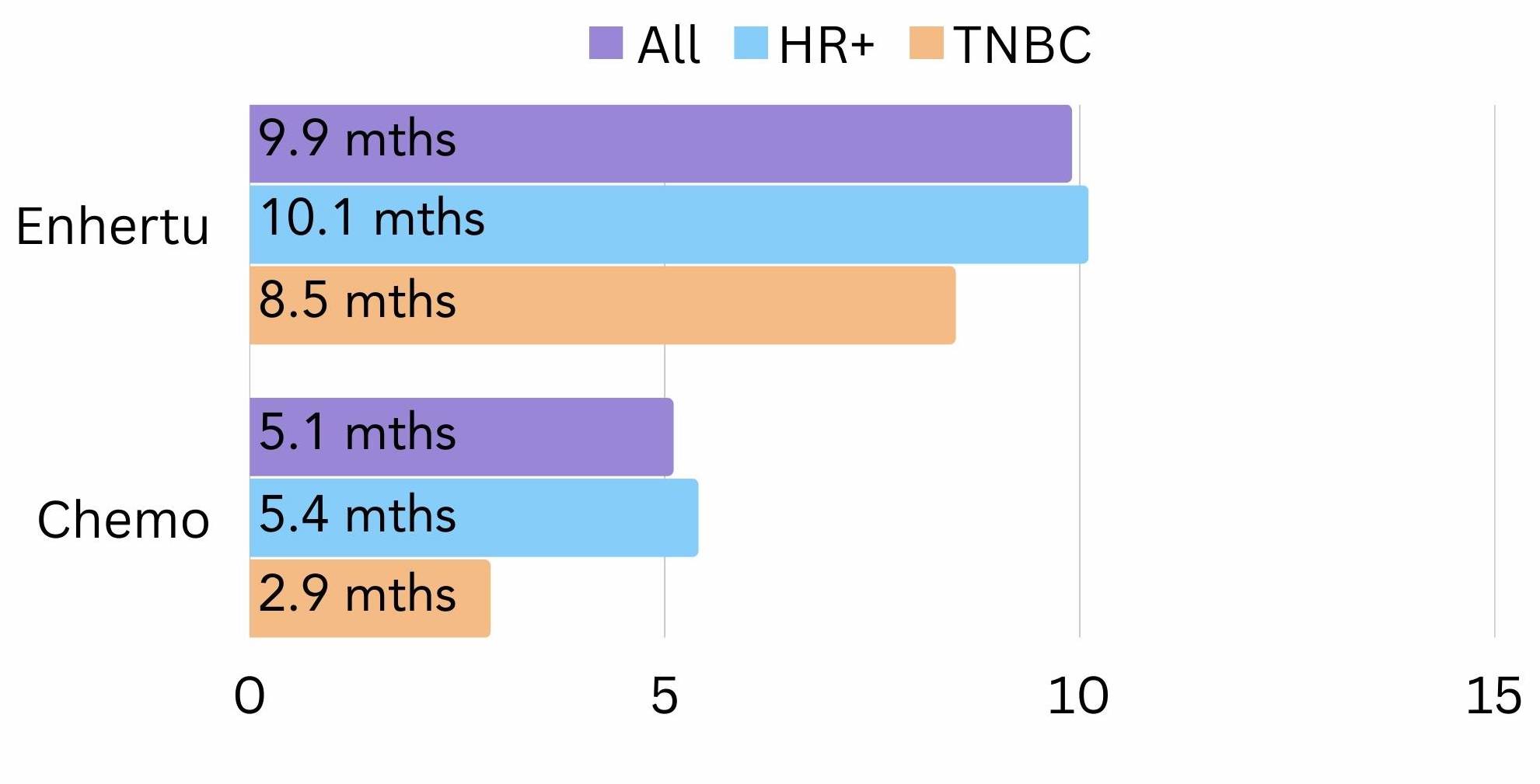
Figure 3. The amount of time all, HR+ and TNBC patients went on average without the cancer growing or spreading (progression free survival) on trastuzumab deruxtecan (Enhertu®) vs chemotherapy in the DESTINY-Breast-04 trial.
How long did HER2-low patients live on trastuzumab deruxtecan (Enhertu®)?
It was also found that most patients on trastuzumab deruxtecan lived 23.4 months longer on average, compared to 16.8 months on chemotherapy12 (figure 4). Those with HR+ breast cancer on trastuzumab deruxtecan lived 23.9 months longer on average, compared to 17.5 months on chemotherapy12 (figure 4). For TNBC patients, those on trastuzumab deruxtecan lived 18.2 months longer on average, compared to 8.3 months on chemotherapy12 (figure 4).
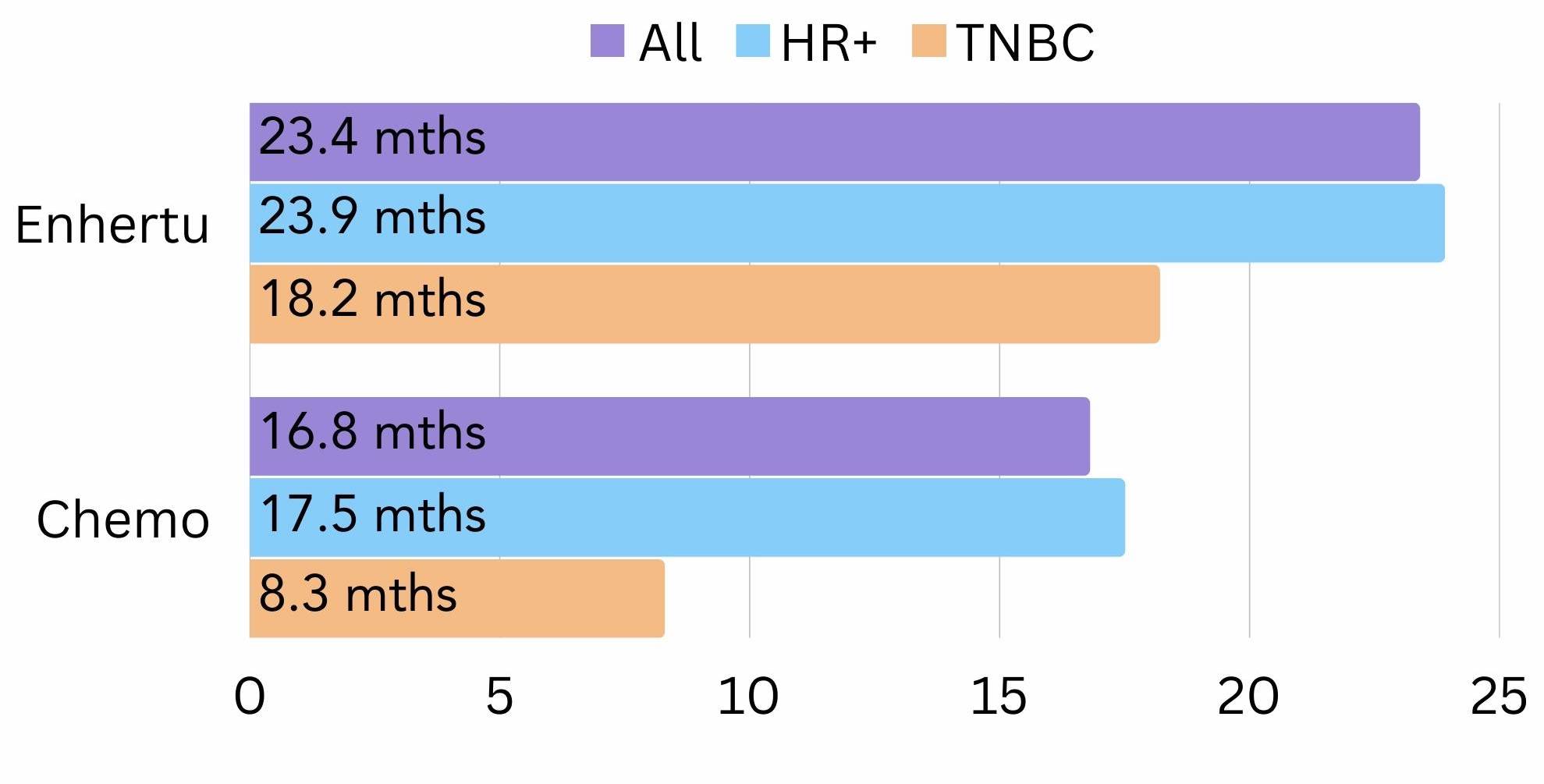
Figure 4. The amount of time all, HR+ and TNBC patients lived for on average (survival) on trastuzumab deruxtecan (Enhertu®) vs chemotherapy in the DESTINY-Breast-04 trial.
Have other HER2 targeted treatments been tested in HER2-low breast cancer patients?
Other monoclonal antibodies have been trialled in HER2-low patients, but the results were limited. Trastuzumab (Herceptin®), pertuzumab (Perjeta®) and margetuximab (Margenza®) were each tested in HER2-low patient populations, it was found that:
- Trastuzumab added to chemotherapy: did not increase the amount of time patients were disease free or improve survival13
- Pertuzumab: the response to treatment was low when used alone. Combined with chemotherapy it offered a slight benefit but was linked to worse side effects14,15
- Margetuximab: was not very effective and many patients had to discontinue due to lack of benefit or side effects, the trial was not completed16
And that’s HER2-low breast cancer summed up
HER2-low is a new distinct type of breast cancer which is characterised by low levels of the HER2 protein. HER2-low can include HR+ and TNBC subtypes with an IHC score of 1+ or 2+ with a negative FISH result. This distinction can provide further understanding of individual tumours and can offer new potential treatment options for this population. Although results are currently limited in monoclonal antibodies, trastuzumab deruxtecan (Enhertu®) is a remarkable ADC that offers a treatment option for HER2-low secondary breast cancer patients in the US and for private patients in the UK. We hope for swift approval of trastuzumab deruxtecan to treat HER2-low patients in the NHS and elsewhere so more HER2-low patients can benefit.
We hope that you now better understand what HER2-low breast cancer is, the impact of it, and the clinical evidence for trastuzumab deruxtecan in HER2-low breast cancer. At OWise, we want to make sure you are kept informed so make sure to follow our Instagram and Twitter for any updates. Any questions? Get in touch!
References
- Li Y, Tsang J, Tam F, Loong T, Tse G. Comprehensive characterization of HER2-low breast cancers: implications in prognosis and treatment. eBioMedicine. 2023;91:104571.
- Cancer Network. Future Outlook for Treatment of HER2-Low and Triple-Negative Breast Cancers. Cancer Network. 2023 Mar 21 [accessed 2023 Aug 9].
- FDA. FDA Approves First Targeted Therapy for HER2-Low Breast Cancer. FDA. 2022 Aug 5. https://www.fda.gov/news-events/press-announcements/fda-approves-first-targeted-therapy-her2-low-breast-cancer
- EMA. Enhertu. EMA. 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/enhertu
- NICE. Trastuzumab deruxtecan for treating HER2-low metastatic or unresectable breast cancer after chemotherapy [ID3935]. www.nice.org.uk. 2023 Nov 15 [accessed 2023 Aug 9]. https://www.nice.org.uk/guidance/indevelopment/gid-ta10813
- Kostova V, Désos P, Starck J-B, Kotschy A. The Chemistry Behind ADCs. Pharmaceuticals. 2021;14(5):442.
- American Cancer Society. Monoclonal Antibody Side Effects | American Cancer Society. www.cancer.org. 2022. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/monoclonal-antibodies.html
- American Cancer Society. How Does Chemo Work? | Types of Chemotherapy. www.cancer.org. 2019 Nov 22. https://www.cancer.org/cancer/managing-cancer/treatment-types/chemotherapy/how-chemotherapy-drugs-work.html
- Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Bioscience Reports. 2015;35(4):e00225–e00225.
- EMA. ENHERTU: SUMMARY OF PRODUCT CHARACTERISTICS. https://www.ema.europa.eu/en/documents/product-information/enhertu-epar-product-information_en.pdf
- Daiichi Sankyo, Inc. and AstraZeneca. Mechanism of Action | ENHERTU® (fam-trastuzumab deruxtecan-nxki) HCP. www.enhertuhcp.com. https://www.enhertuhcp.com/en/gastric/about-enhertu/mechanism-of-action
- Modi S, Jacot W, Yamashita T, Sohn J, Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. New England Journal of Medicine. 2022;387(1).
- Fehrenbacher L, Cecchini RS, Geyer Jr CE, Rastogi P, Costantino JP, Atkins JN, Crown JP, Polikoff J, Boileau J-F, Provencher L, et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy With or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2+. Journal of Clinical Oncology. 2020 [accessed 2022 Aug 15];38(5):444–453.
- Gianni L, Lladó A, Bianchi G, Cortes J, Pirkko-Liisa Kellokumpu-Lehtinen, Cameron D, Miles D, Salvagni S, Wardley AM, Jean-Charles Goeminne, et al. Open-Label, Phase II, Multicenter, Randomized Study of the Efficacy and Safety of Two Dose Levels of Pertuzumab, a Human Epidermal Growth Factor Receptor 2 Dimerization Inhibitor, in Patients With Human Epidermal Growth Factor Receptor 2–Negative Metastatic Breast Cancer. Journal of Clinical Oncology. 2010 [accessed 2023 May 21];28(7):1131–1137.
- Schneeweiss A, Park-Simon T-W, Albanell J, Lassen U, Cortés J, Dieras V, May M, Schindler C, Marmé F, Cejalvo JM, et al. Phase Ib study evaluating safety and clinical activity of the anti-HER3 antibody lumretuzumab combined with the anti-HER2 antibody pertuzumab and paclitaxel in HER3-positive, HER2-low metastatic breast cancer. Investigational New Drugs. 2018;36(5):848–859.
- ClinicalTrials.gov. Phase 2 Study of the Monoclonal Antibody MGAH22 (Margetuximab) in Patients With Relapsed or Refractory Advanced Breast Cancer – Study Results – ClinicalTrials.gov. clinicaltrials.gov. 2022 [accessed 2023 Aug 9]. https://classic.clinicaltrials.gov/ct2/show/results/NCT01828021
