 There is a lot of terminology that comes with a breast cancer diagnosis. And it is easy to become overwhelmed. You can be inundated with different words, treatments and drugs that you have never heard of before, let alone know what they mean. This week we’ll focus on monoclonal antibodies.
There is a lot of terminology that comes with a breast cancer diagnosis. And it is easy to become overwhelmed. You can be inundated with different words, treatments and drugs that you have never heard of before, let alone know what they mean. This week we’ll focus on monoclonal antibodies.
Targeted therapies, those that are designed to target certain characteristics of the breast cancer cell, are filled with long and alien names that would normally be skipped over when reading. However, when it comes to your treatment, we know that having a better understanding of your therapies can help you feel more in control. That’s why we want to give you a little insight into what the drug names actually mean, and from there you can have a better idea of the kind of drug that is being used.
We’re starting with a group of drugs that are used in all breast cancer subtypes. You may have heard of trastuzumab, pertuzumab or even atezolizumab. But what do these drugs all have in common? Those three letters right at the end. “Mab”. This signifies that all these treatments are a form of Monoclonal AntiBody.
How do monoclonal antibodies work?
Antibodies are proteins that make up part of the immune system (the system that protects your body against infection). Antibodies are produced naturally by the body to help the immune system recognise anything that is foreign, for example bacteria and viruses, and then notify the rest of the immune cells to get rid of them. Their specificity means that they have the ability to bind to and identify certain targets. Monoclonal antibodies work using the same concept, except that instead of being produced in the body, these are produced in the lab. And instead of targeting all foreign bodies, they are used to target cancer (check the diagram below).
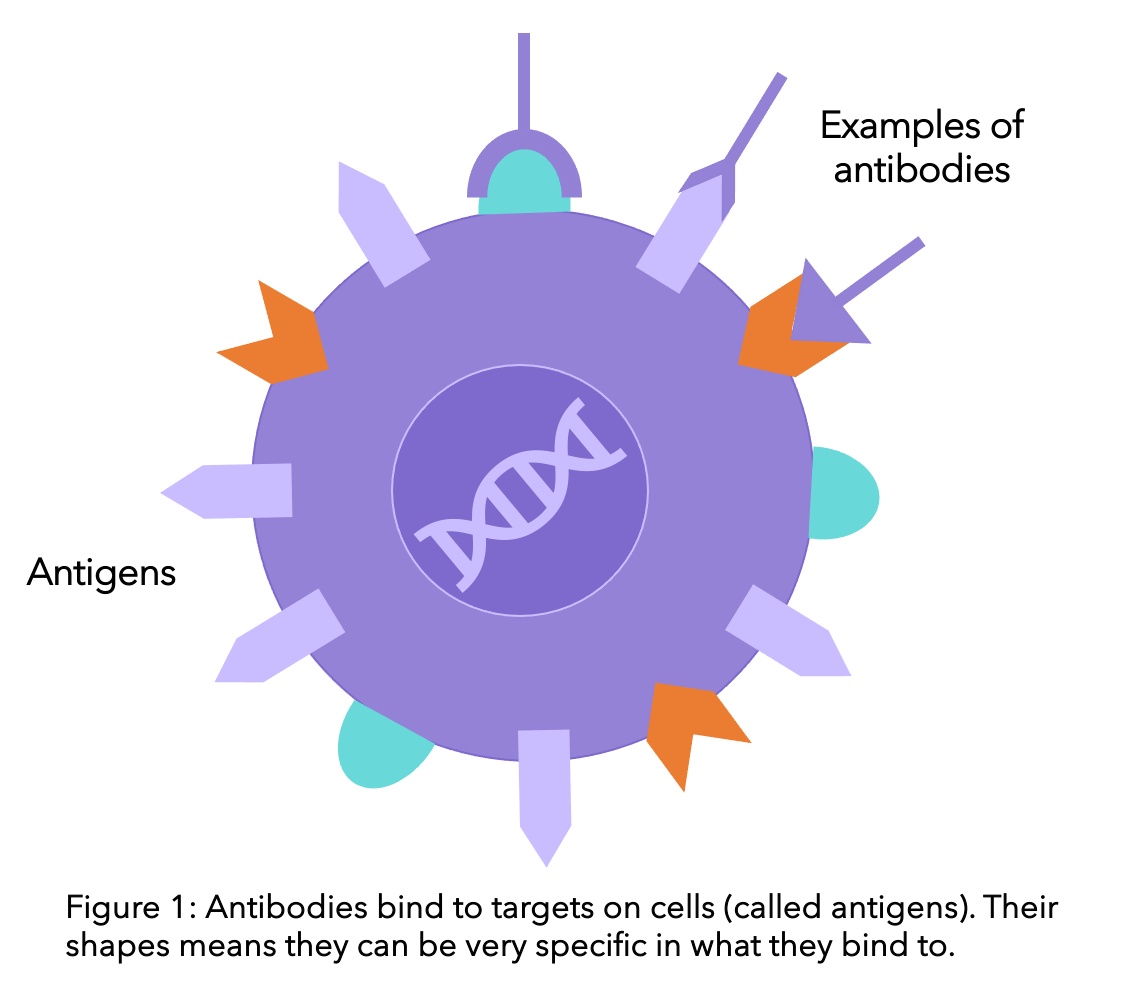
Once the monoclonal antibodies bind to the breast cancer cell, the behaviour of that cell is altered. This usually leads to the destruction of this cell. For example, if the antibody blocks the signals from outside the cell stimulating the cell to grow, growth will cease. Unlike other treatments that have little specificity and can interact with all different types of cells (like chemotherapy interacting with all those that divide quickly), monoclonal antibodies have a very high binding affinity for specific cell surface proteins.
What are the most common side effects caused by monoclonal antibodies?
Due to their high specificity, they do not interact with healthy cells and therefore cause less adverse side effects than other, more generic, treatments, e.g. chemotherapy. However, there are still some you should be aware of.
As they are proteins that are technically deemed foreign by your body, sometimes you may suffer an allergic reaction1. This manifests as
- Fever
- Chills
- Weakness
- Headache
- Nausea
- Vomiting
- Diarrhea
- Low blood pressure
- Rashes
This is common when the drug is first given. If these side effects persist, please speak to your doctor.
Keeping track of your symptoms is easier than ever with the OWise breast cancer app. Using the trends feature you can visualise how your symptoms change over time. Having more information at your fingertips can help you improve communication with your care team and make sure you receive the best care possible. Download the free app today.
Monoclonal antibodies in your treatment plan
For all breast cancer subtypes, there is the opportunity for a monoclonal antibody therapy to be developed. Here we have listed what the most common monoclonal antibodies in breast cancer treatment are and how they work.
For HER2 positive breast cancer
20-25% of breast cancers have high levels of the HER2 protein2. This characteristic can be harnessed for treatment purposes, as you can see below. If you are interested in other treatment options for HER2 positive breast cancer, head to our previous blog!
Trastuzumab (Herceptin®)
If you know about HER2, you have probably also heard of Herceptin®. Created in the 1980’s, Herceptin® was the first targeted therapy to be offered to people with HER2 positive breast cancer. Clinical trials have shown that the introduction of Herceptin® halved cancer recurrence and reduced mortality by 30%3,4,5. But did you know the other name for Herceptin®? Trastuzumab. Yes indeed, the beloved Herceptin® is a star in the monoclonal antibody world.
Herceptin® was the original brand name for the drug, however several similar versions (called biosimilars) are now available. Biosimilars for Herceptin® include Ogivri®, Herzuma®, Ontruzant®, Trazimera®, and Kanjinti®. They are clinically identical, with no changes in effectiveness. However, having multiple options on the drug market allows for the driving down of costs.
But how does it work?
HER2 positive breast cancer cells have an overexpression of HER2 receptors on the surface of their cells. Growth factors can bind to the HER2 receptor, promoting cell growth. Trastuzumab blocks HER2, meaning breast cancer cells no longer receive the signal to grow (check the diagram below).
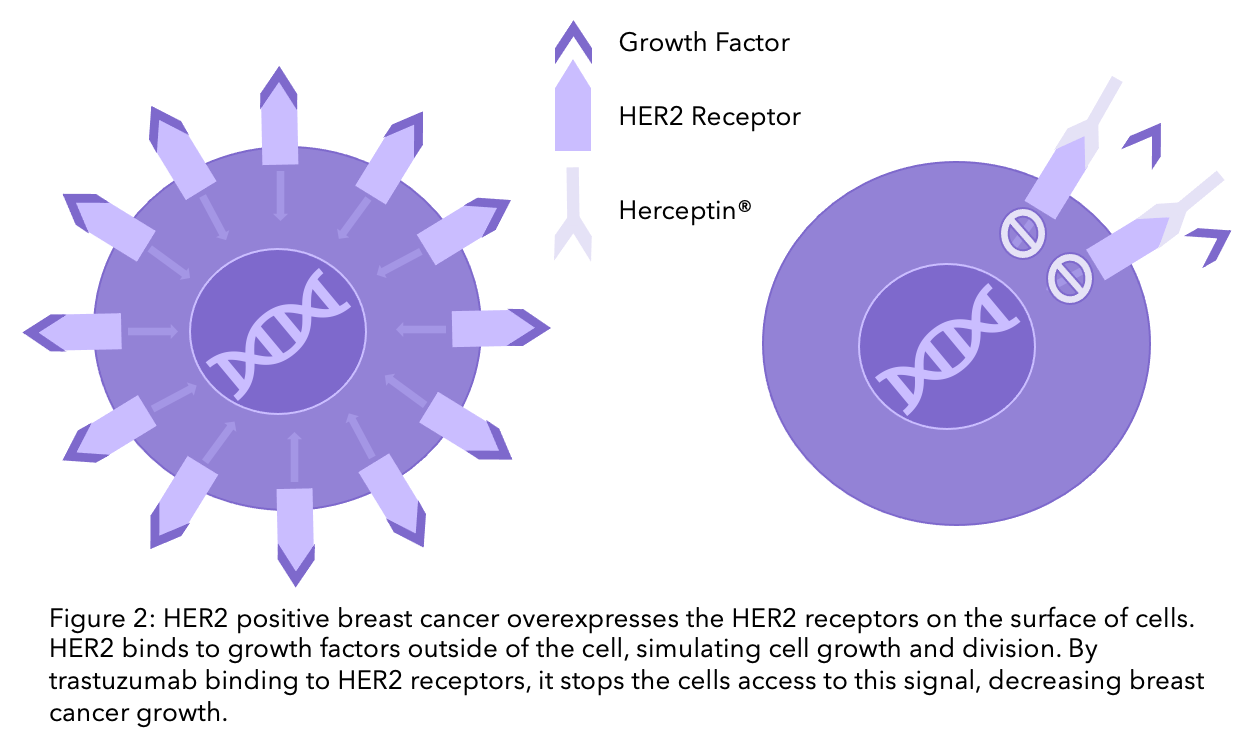
Alternative options including trastuzumab
Trastuzumab can be delivered subcutaneously, meaning with a hyaluronidase injection that goes under the skin rather than through the blood. This has been approved for people with HER2-positive early and advanced breast cancer. There has found to be no difference in the effectiveness of trastuzumab with either method of administration6. Therefore, the decision between the two depends on the individual.
Trastuzumab can also be paired up with a chemotherapy drug. Ado-trastuzumab emtansine (Kadcyla® or TDM-1) is an example of an antibody conjugate (see below) that became available in May 2020.
Pertuzumab (Perjeta®)
Pertuzumab, another monoclonal antibody, can also be used for HER2 positive breast cancer. Pertuzumab works in the same way as trastuzumab, but targets a slightly different area of the receptor. Research has shown that adding pertuzumab alongside chemotherapy and trastuzumab in adjuvant (after surgery) treatment can improve disease-free survival for early breast cancer6.
If you come across any words that you are unsure about in your appointments or when reading up on your diagnosis, OWise is there to help. The app has a glossary which you can refer to at a click of a button whenever and wherever you are, to clarify and understand what certain words mean.
Trastuzumab deruxtecan (Enhertu®)
At the moment, if the second-line therapy (taxane chemotherapy regimen and trastuzumab emtansine) is unsuccessful, there are no further HER2-targeted treatments available. Trastuzumab deruxtecan, brand name Enhertu®, could be the answer. The drug, made up of trastuzumab joined with the chemotherapy agent deruxtecan. It has been approved by NICE and is available for people who have had two previous regimens of targeted therapy that have been unsuccessful. For NHS patients it can be accessed via the Cancer Drugs Fund7 and it is also available via NHS Scotland.
First line therapy refers to the first treatment given for a disease. If this doesn’t cure the disease or causes severe side effects, then second line therapy would be given, and so forth.
If you have triple negative breast cancer
Triple negative breast cancer (TNBC) means that the breast cancer has tested negative for hormone receptors and the HER2 protein. This subtype has historically been trickier to treat, not being able to use targeted therapy like trastuzumab or hormone therapy like tamoxifen, until treatments like monoclonal antibodies came along.
Atezolizumab (Tecentriq®)
Atezolizumab is a monoclonal antibody that works hand in hand with the immune system, making it the first immunotherapy for TNBC in the UK. It relies on the cancer cells testing positive for the PD-L1 protein, which is on the surface of human cells.
PD-L1 proteins are vital in protecting your cells from your immune system. Immune cells have receptors for these proteins and receive a signal that indicates the cell is not dangerous and should not be attacked. Cancer cells use this to their advantage, preventing the immune system from killing them even when they are causing harm. Atezolizumab blocks PD-L1, meaning that the immune system no longer recognises cancer cells as healthy and can then attack them.
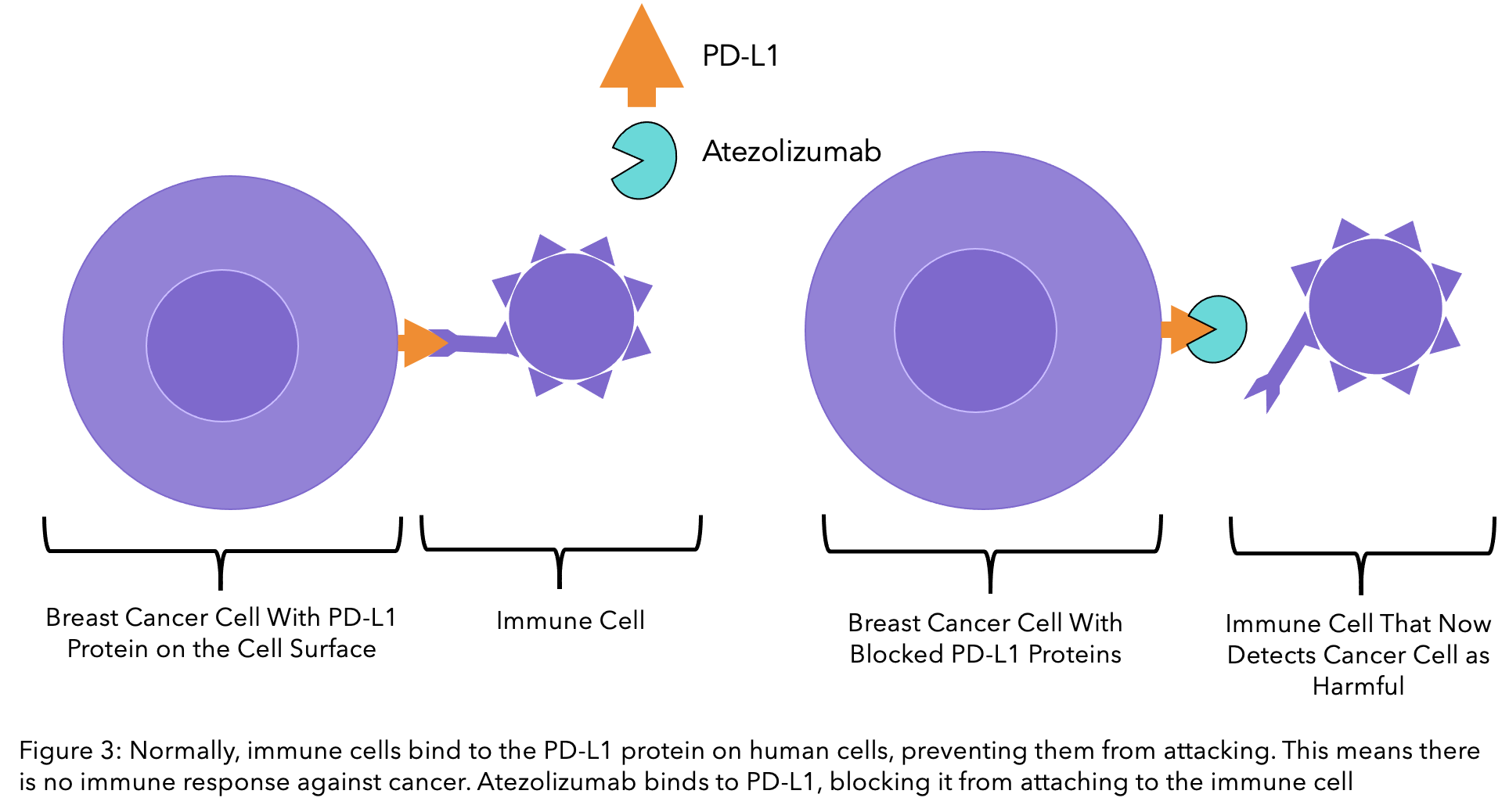
Pembrolizumab (Keytruda®)
As well as targeting PD-L1 on the breast cancer cell, treatments have been made to target the detection system on the immune cell, called PD-1. Pembrolizumab has been created to do just that, and clinical trials have so far been promising9. Although currently unavailable on the NHS, pembrolizumab is being assessed to be used in combination with neoadjuvant chemotherapy (before surgery) with a response expected later this year10.
Find out more about triple negative breast cancer (TNBC) with our blog: TNBC and Me
For hormone receptor positive breast cancer
Hormone receptor positive (HR+) breast cancer is what it says on the tin. Breast cancer that has hormone receptors, and therefore responds to the presence of hormones for growth. For more information about HR+ breast cancer, have a look at our blogs “What’ve hormones got to do with it: Early Stage or Secondary HR+ breast cancer”. There are many treatment options available for hormone positive breast cancer, including tamoxifen and aromatase inhibitors. Until recently, there has been little progress for monoclonal antibodies that target this subgroup. However, that may just be about to change..
Sacituzumab govitecan (Trodelvy®)
A promising drug that could be a feasible treatment option is sacituzumab govitecan (Trodelvy®). This monoclonal antibody targets TROP-2, which is a protein vital for cancer growth. Originally created to be used against triple negative breast cancer, this treatment is an example of an antibody-drug conjugate. This means that the monoclonal antibody that targets TROP-2 is linked to a chemotherapy drug. Once it binds to TROP-2, the chemotherapeutic agent enters the cell and becomes active, killing the cell. Clinical trials are not all complete, but the results so far have been promising, with a study in 2018 finding the response rates comparable to those seen in CDK4/6 inhibitors11. NICE is currently reviewing the treatment with a response expected in June 2022. We endeavour to share the latest updates in regard to available treatment as much as possible. Be sure to check our Twitter and Instagram if you are interested in the future of anti-TROP-2 therapy.
Pembrolizumab (described above for triple negative breast cancer) is currently being trialed to be used for early stage HR+ breast cancer. If you are interested in getting involved, you can find more information on the trial on Cancer Research UK’s page. To find out more about clinical trials and what they could mean for you, head to our previous blog.
A small note on bevacizumab
Bevacizumab is a monoclonal antibody that was originally offered to people with secondary breast cancer as a first-line treatment. It works differently to the other mab’s mentioned so far, blocking the signals induced by a growth factor called VEGF12. VEGF stimulates the growth of blood vessels in a tumour. By stopping their formation of blood vessels, you are essentially starving the tumour of vital nutrients, including oxygen. In a review of the guidance, bevacizumab has been removed as an option for first-line treatment. This is due to cost-effectiveness reasons and other treatments i.e. paclitaxel and docetaxel chemotherapy, are viable alternatives13. You will likely now only receive bevacizumab if you were given it before the change in guidance or as part of a clinical trial.
We hope you now know a little more about monoclonal antibodies and your treatment plan. Keep your eyes peeled for further blogs untangling drug terminology, and if you have special requests, feel free to message us! For more personalised information, download OWise today!
If you want to learn more about your breast cancer type, check out our Breast Cancer Under the Microscope series.
References
-
- Hansel, T., Kropshofer, H., Singer, T., et al. (2010) The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov 9, 325–338.
- Arteaga, C., Sliwkowski, M., Osborne, K., et al. (2011) Treatment of HER2-positive breast cancer: current status and future perspectives. Nat. Rev. Clin. Oncol. 9, 16–32
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. (2006) Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 354(8):809‐820.
- Romond EH, Perez EA, Bryant J, et al. (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 353(16):1673‐1684.
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 353(16):1659‐1672.
- Von Minckwitz,G., Procter, M., de Azambuja, E., et al. (2017) Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. New England Journal of Medicine. 377(7), pp.702-702.
- Nice.org.uk. 2020. Project Information | Trastuzumab Deruxtecan For Treating HER2-Positive Unresectable Or Metastatic Breast Cancer After 2 Or More Anti-HER2 Therapies [ID2697] | Guidance | NICE. [online] Available at: https://www.nice.org.uk/guidance/indevelopment/gid-ta10582 [Accessed 02 February 2022].
- Schmid P, Cortes K, Pusztai L, McArthur H, Kümmel S, Bergh J, Denkert C, Hee Park Y, Hui R, Harbeck N, Takahashi M, Foukakis T, Fasching PA, Cardoso F, Untch M, Jia L, Karantza V, Zhao J, Aktan G, Dent R, O’Shaughnessy J. 2020. Pembrolizumab for Early Triple-Negative Breast Cancer. The New England Journal of Medicine. 382:810-821.
- Nice.org.uk. 2020. Health Technology appraisal. Pembrolizumab in combination with chemotherapy for neoadjuvant treatment of triple negative breast cancer [online]. Available at: https://www.nice.org.uk/guidance/gid-ta10399/documents/draft-scope-post-referral [Accessed 17 January 2021].
- Ismael, G., Hegg, R., Muehlbauer, S., et al. (2012) Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I–III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. The Lancet Oncology, 13(9), pp.869-878.
- Bardia, A. et al., (2018) Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC). Journals of Clinical Oncology. 35(15):suppl, 1004-1004
- Nice.org.uk. 2013. Bevacizumab in combination with a taxane for the first-line treatment of metastatic breast cancer [online]. Available at: https://www.nice.org.uk/guidance/ta214 [Accessed 17 January 2021].
- Nice.org.uk. 2011. Understanding NICE guidance. Bevacizumab in combination with a taxane as first treatment for metastatic breast cancer [online]. Available at: https://www.nice.org.uk/guidance/ta214/resources/bevacizumab-in-combination-with-a-taxane-as-first-treatment-for-metastatic-breast-cancer-pdf-378342541 [Accessed 17 January 2021].
