
Sacituzumab govitecan (Trodelvy®) is a targeted therapy used to treat certain types of secondary (metastatic, advanced) breast cancer. So, let’s delve into the science of Trodelvy and take a look at a secondary breast cancer patient’s experience on this drug. We wrote this blog in collaboration with Make2ndsCount.
Who is Trodelvy for?
Trodelvy for secondary TNBC
Trodelvy is approved to treat triple negative breast cancer (TNBC) that is advanced or cannot be removed by surgery. It is a third line treatment option in the UK, US, and EU, meaning it is a treatment option after two or more prior treatments1,2,3.
Trodelvy for secondary HR+/HER2- breast cancer
In early 2023, Trodelvy was approved to treat hormone receptor-positive/HER2-negative (HR+/HER2-) breast cancer that is advanced or cannot be operated on in the US and EU3,4. It is a third line treatment option for patients who have received hormone (endocrine) therapy, and two or more prior lines of treatment for secondary breast cancer.
What is Trodelvy?
Trodelvy is a type of targeted therapy for breast cancer called an antibody-drug conjugate (ADC). ADCs consist of a monoclonal antibody and chemotherapy drug joined together by a chemical linker5.
Antibodies are proteins that are naturally produced by the body to help fight infection through binding to targets (antigens) that are on the surface of cells6. Monoclonal antibodies are proteins that are made in the lab and are made to act in the same way as natural antibodies.
Scientists can produce monoclonal antibodies that target specific antigens on the surface of cancer cells. This means that the chemotherapy drug that is linked to the monoclonal antibody can be delivered directly to the site of tumour cells7,8. In the case of Trodelvy, the ADC is made up of the monoclonal antibody sacituzumab linked to the chemotherapy agent SN-389 (figure 1).
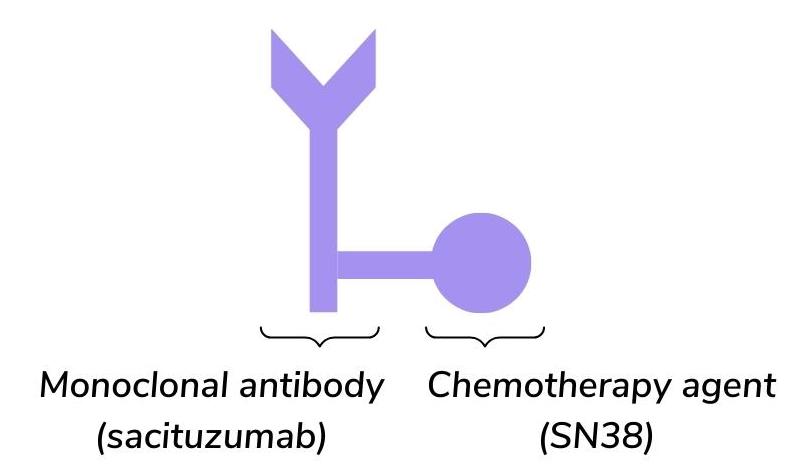
Figure 1. The structure of sacituzumab govitecan (Trodelvy)
How does Trodelvy work?
Certain types of breast cancer cells have a high amount of a protein called trophoblast cell-surface antigen 2 (TROP-2) on their surface. The TROP-2 protein plays a role in cancer developing12.
The sacituzumab monoclonal antibody that is in Trodelvy, targets and binds to TROP-2 proteins, preventing cancer cells from growing and dividing. The SN-38 chemotherapy agent is then taken in (engulfed) by cancer cells, causing cell death. The selective and precise nature of Trodelvy enables it to work as highly targeted drug, this prevents healthy cells from being impacted and therefore, fewer side effects.
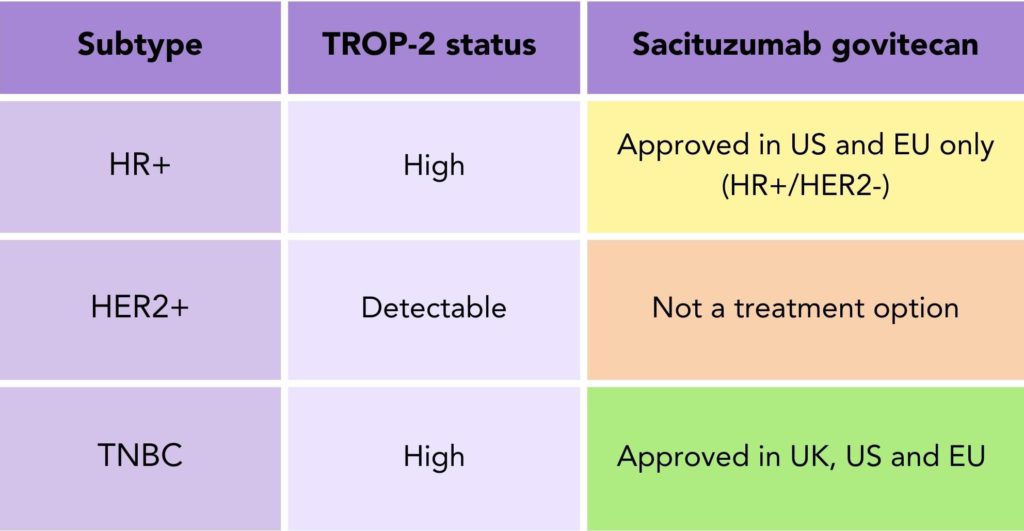
Research suggests that the TROP-2 protein is seen across all breast cancer subtypes, but it is seen in higher amounts in TNBC and HR+/HER2- breast cancer subtypes11 (table 1). Please note that TROP-2 levels are not measured for Trodelvy to be prescribed.
Table 1. Sacituzumab govitecan (Trodelvy®) approval status to treat secondary breast cancer by subtype
What clinical evidence is there for Trodelvy in treating breast cancer?
ASCENT (TNBC)
The ASCENT trial compared Trodelvy with single-agent chemotherapy (eribulin, vinorelbine, capecitabine, or gemcitabine) in 529 patients with secondary TNBC. Of these patients, 61 of them showed evidence of having brain metastases12.
In the group without brain metastases, most patients on Trodelvy went on for 5.6 months without the cancer growing or spreading, compared to 1.7 months on chemotherapy (figure 2).
Results also showed that over a 24-month period, most patients without brain metastases lived, on average, for 12.1 months on Trodelvy, compared to 6.7 months on chemotherapy (figure 2).
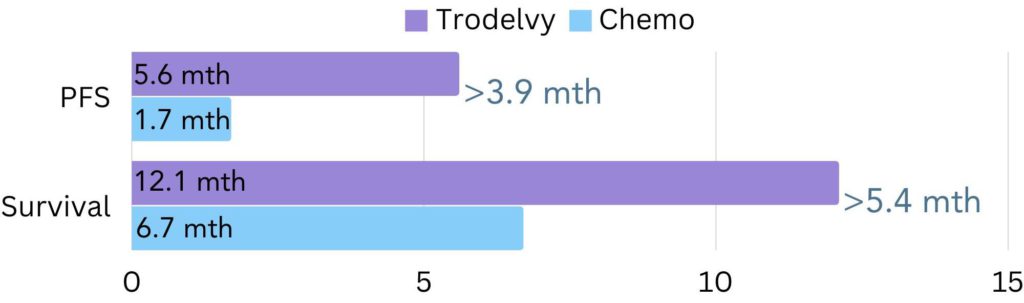
Figure 2. The amount of time patients without brain metastases went on average without the cancer growing or spreading (progression free survival/PFS) and lived for (survival) in the ASCENT trial. The difference in PFS between Trodelvy and chemotherapy was 3.9 months and the difference in survival was 5.4 months.
Patients on Trodelvy had increased progression free survival and overall survival compared to patients on chemotherapy.
Todelvy patients had a progression free survival 3.9 months longer than chemotherapy patients and survival was 5.4 months longer.
When looking at data from the full patient population, including those with brain metastases, it was found that for most patients on Trodelvy the cancer did not grow or spread for 4.8 months, compared to 1.7 months on chemotherapy (figure 3). Additionally, over a 24-month period, most patients lived 11.8 months on average on Trodelvy, compared to 6.9 months on chemotherapy (figure 3).
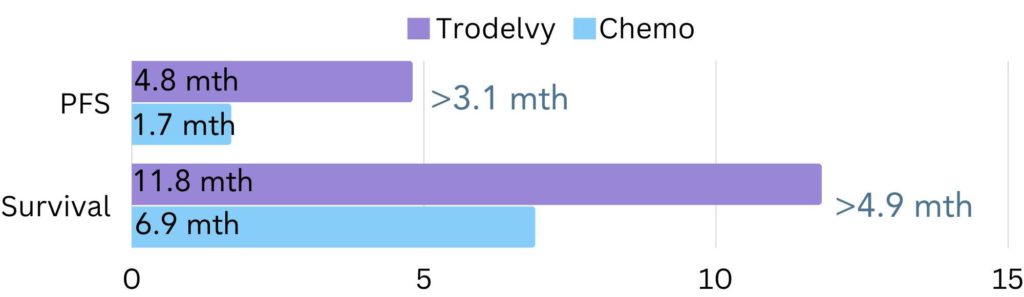
Figure 3. The amount of time all patients went on average without the cancer growing or spreading (progression free survival/PFS) and lived for (survival) in the ASCENT trial. The difference in PFS between Trodelvy and chemotherapy was 3.1 months and the difference in survival was 4.9 months.
TROPiCS-02 (HR+/HER2-)
The TROPiCS-02 trial compared Trodelvy with single-agent chemotherapy (capecitabine, eribulin, vinorelbine, or gemcitabine) in 543 patients with HR+/HER2- breast cancer that could not be operated on, was locally advanced or advanced13.
Evidence showed that most patients went on without the cancer growing or spreading for 5.5 months on Trodelvy, compared to 4 months on chemotherapy (figure 4). Additionally, patients lived for 14.4 months on average on Trodelvy, compared to 11.3 months on chemotherapy (figure 4).
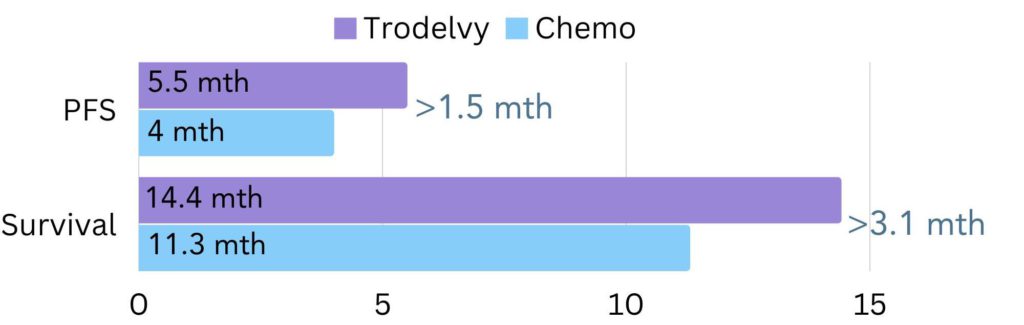
Figure 4. The amount of time patients went on average without the cancer growing or spreading (progression free survival/PFS) and lived for (survival) in the TROPiCS-02 trial. The difference in PFS between Trodelvy and chemotherapy was 1.5 months and the difference in survival was 3.1 months.
What side effects can Trodelvy cause?
Like any medication, Trodelvy can cause side effects. Common side effects include loss of appetite, hair loss, headaches, muscle and joint pains, nausea and vomiting, and fatigue14. There are also more serious side effects that should be monitored and reported to your care team, including low blood cell count (neutropenia), diarrhoea and allergic reaction14.
Low white blood cell count
Neutropenia, also known as low white blood cell count, can sometimes occur while on Trodelvy treatment. Low levels of white blood cells can make it harder for your body to fight infections, which can increase susceptibility to infectious diseases. It is important contact your care team if you develop14:
- Fever
- Chills
- Cough
- Shortness of breath
- Burning or pain when urinating
- Other signs of infection
Neutropenia can be managed by regular monitoring of white blood cell counts. If necessary, there are treatment options available that can increase white blood cell count15. In the case of infection, antibiotics, white blood cell transfusions and stem cell transplants are possible courses of action.
Diarrhoea
Diarrhoea can be a common side effect of Trodelvy treatment and is characterised by having loose stools three or more times over a 24-hour period16. It needs to be monitored as severe diarrhoea can lead to dehydration and kidney problems. Contact your care team if14:
- You get diarrhoea for the first-time during treatment with Trodelvy
- Your stool is black or bloody
- You feel lightheaded, dizzy or faint
- You are unable to retain fluid due to nausea and vomiting
- Your diarrhoea lasts longer than 24 hours
Your care team can give you medicine to control the diarrhoea and can give you fluids and electrolytes to rehydrate you. If the diarrhoea cannot be controlled with medication your care team may choose to reduce your dose of Trodelvy or stop treatment.
Allergic reaction
Trodelvy can cause allergic or infusion related reactions, although not common, this can be serious and symptoms that develop in the first 24 hours after your infusion should be reported to your care team immediately14:
- Swelling of the face, lips, tongue or throat
- Skin rash
- Itching
- Hives
- Flushing of the skin
- Fever
- Finding it difficult to breath or wheezing
- Feeling lightheaded, dizzy or faint
- Chills or shaking chills
Have you tried tracking your side effects on OWise? With our easy-to-use sliders, you can track over 30 different side effects. This makes it simple to monitor and track key side effects you need to look out for. You can even share your trends directly with your care team and loved ones.
Lesley Morris’ Story: Breast cancer diagnosis to Trodelvy
What is your secondary breast cancer diagnosis background?

I was diagnosed with triple negative breast cancer in January 2022 and went through chemo; EC followed by carboplatin and paclitaxol, single mastectomy and radiotherapy. About six weeks after my mastectomy, I noticed a red patch on the scar line which was subsequently biopsied and confirmed as cancerous. I had radiotherapy as planned which successfully treated the area and I then had Capecitabine to ‘mop up’ any rogue cells.
I had two clear scans but unfortunately my next scan in February 2023 showed spread to some lymph nodes in the chest and collarbone area. It was at this point my oncologist advised me that she does not think my cancer is curable. The affected nodes are not in an area that can be treated with further radiotherapy or surgery, so I began sacituzumab govetican (Trodelvy) in May 2023.
What has been your experience on Trodelvy?
I started on a 50% dose of Trodelvy due to poor tolerance of previous chemo. I am finding the 50% dose of Trodelvy fairly manageable. The main side effect has been hair loss which has been tough. I have always had long hair and lost it during primary treatment. When it came back in it was curly and quite unruly, think Bob Dylan but on a bad day! The Beatson Cancer Charity have an excellent hairdressing service for patients and after an appointment with their fabulous stylist I loved my new hair, so I was really upset to lose it again. I have a wig that I like and if you don’t know me you wouldn’t know that it’s a wig but it’s just not the same and I miss my crazy curls.
What other side effects do you experience on Trodelvy?
Other side effects are constipation, which I think is more from the pre-meds than Trodelvy itself and some fatigue. The constipation I manage mainly through diet, so plenty of fruit, veg, fibre and some laxatives to help things along if need be. A top tip I got from a dietitian on relieving constipation is to eat kiwi fruit.
I’m now in a routine with this treatment so I know that a Monday is my worst day in terms of fatigue. To manage it I pace myself and try to do things in the morning when I feel ok and rest in the afternoon if I need to.
It may seem counterintuitive, but I find that exercise really helps with fatigue and although I don’t always feel like it, if I go for a half hour walk I feel much better for it afterwards. I’ve also found that being disciplined with myself and going to bed earlier rather than watching rubbish on TV or mindlessly scrolling through instagram helps me manage the fatigue.
My treatment has kicked me into an early menopause which brings with it hot flushes and night time visits to the loo, so I really feel it the next day if I stay up too late. I’m very keen on using alternative therapies to manage treatment side effects and enhance my wellbeing. I take various supplements, exercise regularly and eat a healthy balanced diet (with occasional treats or meals out) and try to get 8 hours of sleep every night (hot flushes and bladder permitting!).
I’ve read a lot about the benefits of mindfulness and meditation but I really struggle to empty my busy grasshopper mind long enough to get much out of it, but I’ll keep persevering. I go for a walk every day regardless of the weather and I really enjoy being out in nature hearing the birds and watching the trees change with the seasons. That might be as close to mindfulness as I’ll get!
Have you had any scans starting Trodelvy?
My first scan since starting Trodelvy was stable and I’m keeping everything crossed that the next scan shows an improvement. I’ve seen some very positive stories from people on this treatment with some achieving no evidence of disease.
I really believe that an integrative approach to complement the treatment is helping me keep well, hopefully for a very long time to come.
And that’s Trodelvy summed up
Trodelvy is such an exciting advancement in the treatment of secondary breast cancer, particularly for patients with secondary TNBC and HR+/HER2- breast cancer. With its targeted and precise approach, this antibody-drug conjugate offers improved outcomes for patients who have exhausted other treatment options. Although it may come with potential side effects, these can mostly be managed with the support of your healthcare team.
For more on this series check out ‘Secondary breast cancer: pathology, signs and diagnosis‘, ‘Enhertu for Secondary Breast Cancer: Science and Stories‘ and the Make2ndsCount website for more on the emotional impact of secondary breast cancer.
At OWise, we want to make sure you are kept informed so make sure to follow our Instagram and Facebook for any updates. Any questions? Get in touch!
References
- NICE. Sacituzumab govitecan for treating unresectable triple-negative advanced breast cancer after 2 or more therapies. www.nice.org.uk. 2022. https://www.nice.org.uk/guidance/ta819
- FDA. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. FDA. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-sacituzumab-govitecan-triple-negative-breast-cancer
- EMA. Trodelvy . European Medicines Agency. 2021. https://www.ema.europa.eu/en/medicines/human/EPAR/trodelvy
- FDA. FDA approves sacituzumab govitecan-hziy for HR-positive breast cancer. FDA. 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-sacituzumab-govitecan-hziy-hr-positive-breast-cancer
- Koster K-L, Huober J, Joerger M. New antibody-drug conjugates (ADCs) in breast cancer—an overview of ADCs recently approved and in later stages of development. Exploration of Targeted Anti-tumor Therapy. 2022;3(1):27–36. doi:https://doi.org/10.37349/etat.2022.00069
- American Cancer Society. Monoclonal Antibody Side Effects | American Cancer Society. www.cancer.org. 2022. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/monoclonal-antibodies.html
- Kostova V, Désos P, Starck J-B, Kotschy A. The Chemistry Behind ADCs. Pharmaceuticals. 2021;14(5):442. doi:https://doi.org/10.3390/ph14050442
- Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Bioscience Reports. 2015;35(4):e00225–e00225. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4613712/. doi:https://doi.org/10.1042/bsr20150089
- Breast Cancer Now. Sacituzumab govitecan (Trodelvy). Breast Cancer Now. https://breastcancernow.org/about-breast-cancer/treatment/targeted-biological-therapy/sacituzumab-govitecan-trodelvy/
- Zaman S, Jadid H, Denson AC, Gray JE. Targeting Trop-2 in solid tumors: future prospects. OncoTargets and Therapy. 2019;12:1781–1790. doi:https://doi.org/10.2147/ott.s162447
- Vidula N, Yau C, Rugo HS. Trop2 gene expression (Trop2e) in primary breast cancer (BC): Correlations with clinical and tumor characteristics. Journal of Clinical Oncology. 2017;35(15_suppl):1075–1075. doi:https://doi.org/10.1200/jco.2017.35.15_suppl.1075
- Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, Brufsky A, Sardesai SD, Kalinsky K, Zelnak AB, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. New England Journal of Medicine. 2021;384(16):1529–1541. doi:https://doi.org/10.1056/nejmoa202848
- Rugo HS, Bardia A, Tolaney SM, Arteaga C, Cortes J, Sohn J, Marmé F, Hong Q, Delaney RJ, Hafeez A, et al. TROPiCS-02: A Phase III study investigating sacituzumab govitecan in the treatment of HR+/HER2- metastatic breast cancer. Future Oncology. 2020;16(12):705–715. doi:https://doi.org/10.2217/fon-2020-0163
- Gilead Sciences. TRODELVY® (sacituzumab govitecan-hziy). Trodelvy. https://www.trodelvy.com
- American Cancer Society. Low White Blood Cell Counts | Neutropenia. www.cancer.org. 2023. https://www.cancer.org/cancer/managing-cancer/side-effects/low-blood-counts/neutropenia.html
- Cancer Research UK. Causes of diarrhoea | Coping physically | CancerResearch UK. www.cancerresearchuk.org. 2022. https://www.cancerresearchuk.org/about-cancer/coping/physically/bowel-problems/types/diarrhoea/causes#:~:text=Some%20chemotherapy%20drugs%20irritate%20the
