Today’s research is tomorrow’s treatment
– MetUp UK1
Around the world today there are over 2000 clinical trials focusing on breast cancer that are open to recruitment2. They are the final step in the grand scheme of advancing medicine and giving people the opportunity to receive the most innovative treatments. Without clinical trials, crucial discoveries in healthcare would not transform into accessible options available to people on the NHS. This was how Herceptin® (trastuzumab), which was first studied in the 1990s, could become so widely available. Since its first clinical study that showed it was effective against HER2 positive breast cancer, it has been shown to halve the rate of recurrence and reduce mortality by 30%3,4,5.
Becoming part of a clinical trial can be an immensely rewarding experience, giving you the opportunity to contribute to important research. However, clinical trials do come with their own risks, and so it is important to have a balanced view towards them. That’s why we have created this comprehensive guide. Here we will explore the crowded landscape of clinical trials and provide the most up to date information about what they are, how you can get involved, and answers to common questions that arise. Do remember though, taking part in a clinical trial is completely up to you. We just want to provide you with all the information you need to make an informed choice.
Every advance in cancer treatment in recent years has come out of a clinical study. Without clinical trials, new therapies could not be approved, so clinical trials are essential for advancing medical progress.
Yet, only 15% of cancer patients are aware of clinical trials as a treatment option and less than 5% take part in clinical trials. This low participation rate can be explained by many reasons, one of them being the lack of information about clinical studies and the difficulties that patients face when navigating the clinical trials jungle in order to find a study suitable for their needs and preferences.
– Sara Liyanage, Ticking off breast cancer in collaboration with Ancora.ai6
Firstly, what is a clinical trial?
A clinical trial is an umbrella term for various studies that take place in order to test whether a new treatment or pathway could be of significant benefit when incorporated into standard practice. They are the final stage of a long research process which would have all started in the lab.
Clinical trials are crucial for the development of our healthcare system. They are vehicles that allow for new treatment options to become available. Most recently in the world of breast cancer treatment, clinical trials translated into the approval of atezolizumab (Tecentriq®), the first approved immunotherapy for people with triple negative advanced cancer7.
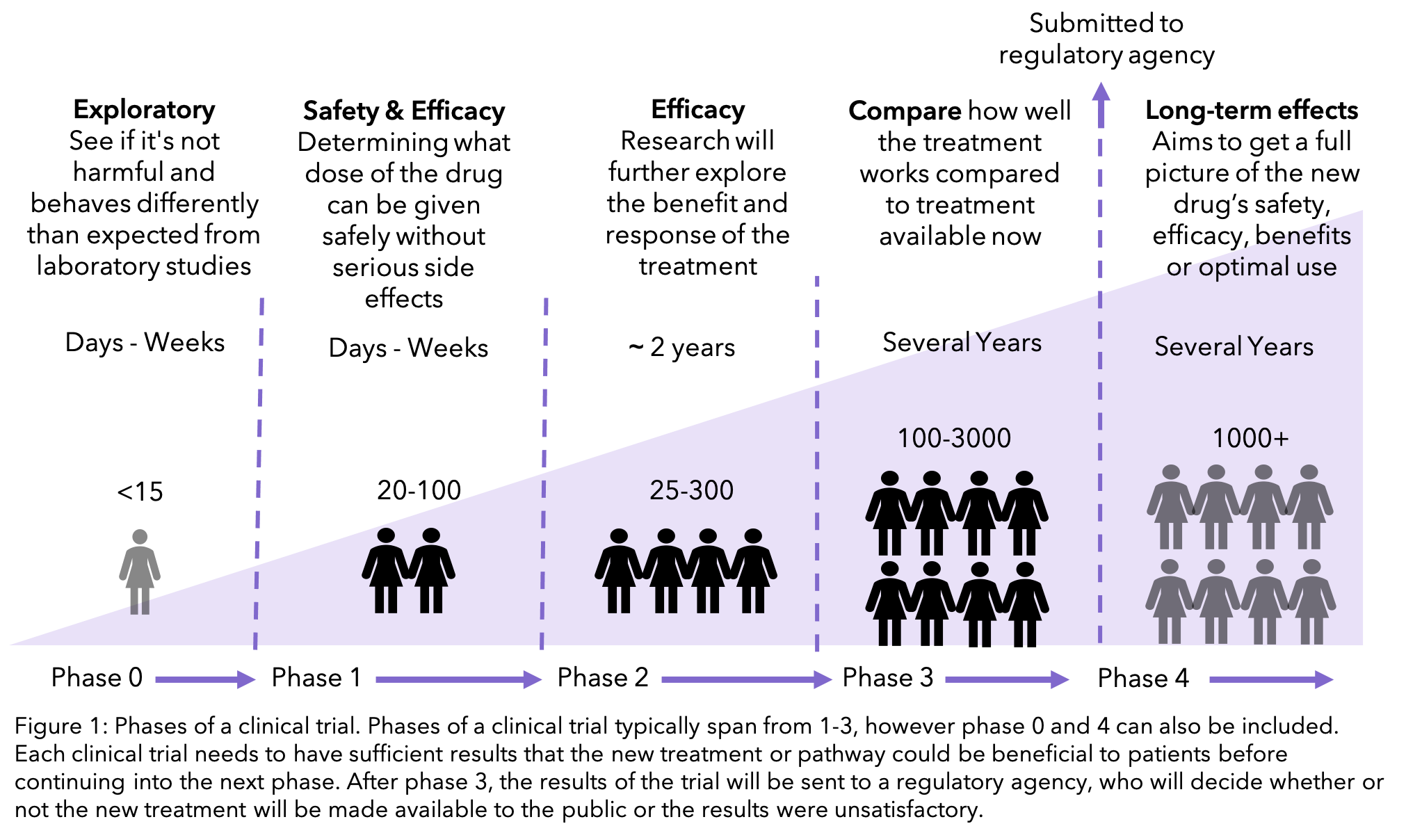
New treatments go through multiple stages of clinical trials, called phases, which get larger and more widely available the more they learn about the treatment. If at an early phase it is clear that the treatment would not be beneficial to patients, then all further research of the treatment is suspended.
ROLo is a phase 2 trial for people with secondary breast cancer. It is looking at the use of a new targeted therapy called crizotinib that blocks messengers that tell the cancer to grow. This trial is available for people with ER positive, HER2 negative breast cancer. Find out more here.
Concerning breast cancer, clinical trials span from prevention, detection, and treatment. In this blog we will be focusing on clinical trials that cover treatment for both early and late stage breast cancer. They can materialize as a test for a new treatment, or it can study social aspects, for example looking at the percentage of women that struggle with fear of recurrence and methods to manage it.
The most important question: are clinical trials safe?
The answer to this isn’t straight forward. Most clinical trials focus on treatments that are currently not available to the general public. Prior to being studied through a clinical trial, these treatments will have gone through rigorous testing, normally in human cells in the laboratory and animal models of the disease. Any findings will then have to be thoroughly reviewed by independent teams of scientists and a research ethics committee8.
Only after this process of review can the trial recruit patients. If they believe the drug could do more harm than good, the clinical trial will not go forward. As described earlier, clinical trials go through multiple phases. The earliest phase will be exploring whether the theory that was validated in the laboratory is also the case in people. This will be the phase where there is the least amount of knowledge of any adverse effects that there may be. The later phases of a clinical trial will focus on whether the medication is better than the current available treatment, as well as determining the long-term impacts9.
This does not mean that earlier phases are dangerous. The process of clinical trials means that the first patients tested will receive very small doses and the effects measured extensively before higher doses are administered.
As well as this you will always have the option to stop taking part at any point within the clinical trial.
So, are clinical trials safe? The answer is that authorities that approve clinical trials do everything in their power to make sure that clinical trials are as safe as possible. However, as the treatment has not been tested in people before, there may be adverse effects that had previously not been considered. You will always be told the potential risks and benefits of the trial before enrolling.
What type of clinical trials can you take part in?
Interventional trials
Interventional trials are used to test a new treatment, either against the best current treatment available or a placebo (see below). These are split into the phases that were described earlier. The higher the number of the phase, the larger the trial is and the more likely that you would be able to join it. Phase 1 trials are normally offered to people with secondary breast cancer by their doctors, typically when they have already had all the standard treatments available. Phase 2 and 3 trials are much larger, and you are able to ask to join. To find out what phase may be right for you, head to Ancora.ai’s post outlining the pros and cons for each.
Randomised controlled trials (RCTs)
RCTs are the main design of an interventional trial. It works by comparing the new option against the best current treatment or placebo. Randomised refers to the fact that neither you nor your care team can decide which group of the trial you will be in. Instead, trial managers use computer programs that randomly allocate you to either of the groups. This is to make sure that the results are reliable. The comparison between the two groups will be used to evaluate the new treatment.
If you receive the standard care, you will be part of the ‘control’ group. This can be difficult to face as many would join the trial hoping to receive the new treatment. However, the role of a control is vital, otherwise the effect of the new treatment cannot be accurately assessed and new treatments could not be incorporated into standard care.
KEYNOTE-756 is a phase 3 RCT that is looking at adding pembrolizumab to chemotherapy and hormone therapy for newly diagnosed breast cancer. This new therapy is an immunotherapy that targets PD-1 on immune cells. It is offered to people who are oestrogen positive, HER2 negative. For more information, head to Cancer Research UK’s website.
Placebo
 A placebo is a drug that has no active ingredients but is made to look like the drug being tested. This is sometimes used in RCTs to compare against a new treatment being offered. The aim of the placebo is to make the environment the same in both groups of the trial. This means that the results seen can be assumed to be caused by the new treatment rather than an external factor.
A placebo is a drug that has no active ingredients but is made to look like the drug being tested. This is sometimes used in RCTs to compare against a new treatment being offered. The aim of the placebo is to make the environment the same in both groups of the trial. This means that the results seen can be assumed to be caused by the new treatment rather than an external factor.
Please note this does not mean you will not receive any treatment. You will still receive the standard care offered by the NHS. Placebos are needed when there is no standard of care comparable to the new treatment and therefore cannot be tested against each other.
Some trials will not have any placebos at all, and others may use different techniques depending on the nature of the trial. For example, if it were testing a new surgery procedure, the control group would have a sham procedure, where the patient goes through the process of having surgery without any surgery taking place.
Did you know about the placebo effect? This is when people who receive a placebo seem to do better both psychologically and physically. This is thought to be due to the relationship between the mind and body. A common theory is that a person expects a pill to do something, then it is possible that the body’s own chemistry can cause similar effects to what the medication would have caused10.
Double blind
Normally you do not know whether you are receiving the active treatment or a placebo. If your doctor also does not know, then the trial is called double blind. This is used to prevent bias in the study, e.g. a doctor involved in the trial being more vigilant about documenting side effects if you were on the active treatment.
CAPItello 290 is a phase 3 trial offered to people with triple negative breast cancer that has come back or spread to another part of the body. It is studying whether a new targeted therapy called capivasertib could be beneficial alongside paclitaxel. Volunteers will either be given capivasertib or a placebo with their chemotherapy. Find out more here.
Observational trials
The above terms are all related to interventional trials. Clinical trials that are looking at other aspects such as quality of life and do not have an effect in your day-to-day, are defined as observational. These trials do not go into as much rigorous testing as the interventional trials as they should not have a direct impact into your life.
Where can I find a trial that’s right for me?
Cancer Research UK has created an extensive resource for clinical trials, including a database showing all trials that are currently recruiting for breast cancer, which can be divided into phase, trial type (e.g. treatment, side effects, other factors) and location.
The NIHR has also created the campaign Be Part of Research, to increase accessibility of clinical trials to the general population. They have created a UK database that can be split into early and advanced breast cancer clinical trials.
“I’ve learned that my doctor doesn’t know about all of the clinical trials….I see clearly that it is my responsibility as a patient to ensure that I keep my eyes open for options that I uniquely qualify for and would uniquely benefit from.”
Each clinical trial often has an extensive list of inclusion and exclusion criteria. These are usually based on medical history, diagnosis and other factors. It may therefore be the case that you have the right sub type or stage of breast cancer, but you are not eligible for the trial. Please do not be disheartened by this. The inclusion and exclusion criteria are created with patient safety in mind to ensure patients will not be put under harm by being part of the trial.
Clinical trials are located all over the UK. Make sure to check the location before deciding if the trial is for you. You can also focus your searches to your local area in the trial databases. You will most likely have to travel to the clinical trial site multiple times whilst taking part, so make sure you take this into account when you’re making a decision. Although most trials reimburse you for the cost of travel, it is still advised to double check with the trial coordinator.
What’s in it for you?
Being part of a clinical trial can be an immensely rewarding experience. The research that you are a part of can change the future of healthcare as well as benefit you personally. Firstly, you may have the opportunity to access a new treatment that is not available to people outside the trial. There has also been research showing that people who take part in clinical trials tend to do better overall, most likely due to the fact there is more access to the healthcare team and you are watched more closely12,13. In secondary breast cancer, many of the treatments being studied focus on targeted therapies, which often have fewer side effects than traditional chemotherapy1.
Are there any risks you should be aware of?
Although uncommon, there are some trials where you don’t receive the standard treatment along with the new one. This has the risk that you may receive a less beneficial treatment. As explained previously, the earlier the phase, the less knowledgeable the research team is about adverse effects of the treatment. Therefore you may experience more severe side effects than you would have with standard care. As well as this, whilst some of the follow ups will be via telephone, some may be in person. This means you will need to incorporate more appointments and tests into your daily life, along with the potential incurred cost.
“[Clinical trials] open another door of possibility and treatment options in conjunction with your current treatment. Is it nerve-wracking and uncertain? Yes, but so is a cancer diagnosis. It is so important to be armed with knowledge and clear away the misconceptions about clinical trials. In the end, it could help save your life or prolong your quality of life.”
– Megan-Claire Chase, in her piece published in Elephants and Tea Magazine14
What should I do if I want to be part of a clinical trial?
If you are interested in taking part in a clinical trial, you should discuss this with your oncologist. They will be able to discuss clinical trials that are currently taking place, or, if you found one you would like to join, discuss with you the specifics of that trial in particular. You can ask to go through the inclusion and exclusion criteria to make sure the trial is suitable for you.
If you decide to join a trial, you will be asked to sign an informed consent document. This documents that the trial organisers have shared all the details of the trial and that you understand these details and accept them. This is not a binding contract for you to stay in the trial. You can leave a trial at any point, with no need to give a reason.
How OWise can support you when you’re in a clinical trial
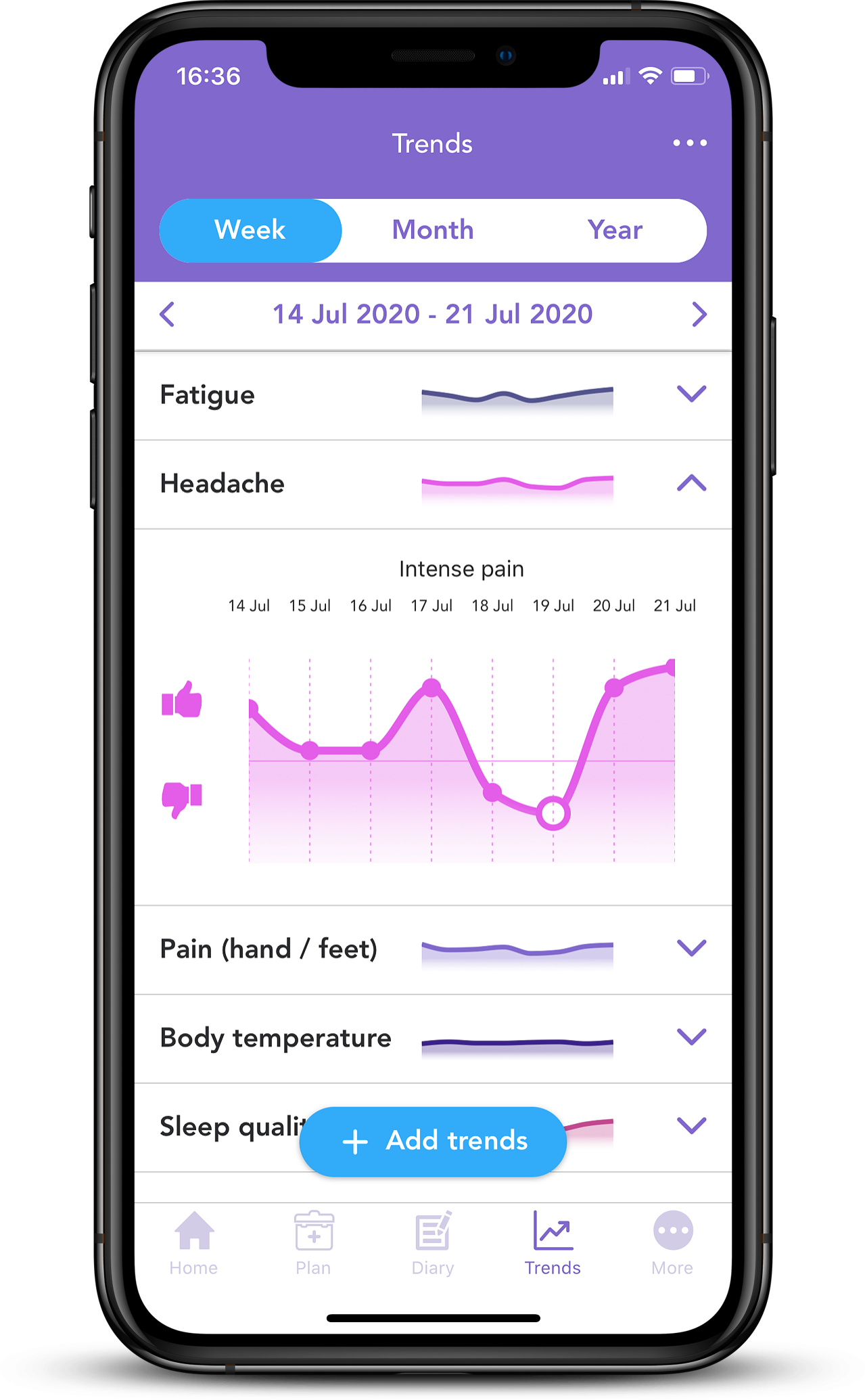 OWise has been created to help all people going through a breast cancer diagnosis and treatment. During a clinical trial, it is crucial to understand the side-effects you are experiencing. If they are particularly difficult for you, you always have the choice to withdraw from the trial and return to standard care. As well as this, as the treatment is still in the testing stage, there may be side effects that the leads of the clinical trial may not be aware of. That’s why it’s crucial to track any side effects that you are experiencing and monitor how they change over time. This will give you important insights into how the treatment is affecting you. There can be a ‘hype’ around clinical trials, with the expectation that you are receiving the best and most innovative treatment to date. Sometimes it can be difficult to question whether this new treatment may not be beneficial to you. By tracking side effects and noting their severity, you can discuss this with your care team and decide whether continuing in the trial is the best thing for you.
OWise has been created to help all people going through a breast cancer diagnosis and treatment. During a clinical trial, it is crucial to understand the side-effects you are experiencing. If they are particularly difficult for you, you always have the choice to withdraw from the trial and return to standard care. As well as this, as the treatment is still in the testing stage, there may be side effects that the leads of the clinical trial may not be aware of. That’s why it’s crucial to track any side effects that you are experiencing and monitor how they change over time. This will give you important insights into how the treatment is affecting you. There can be a ‘hype’ around clinical trials, with the expectation that you are receiving the best and most innovative treatment to date. Sometimes it can be difficult to question whether this new treatment may not be beneficial to you. By tracking side effects and noting their severity, you can discuss this with your care team and decide whether continuing in the trial is the best thing for you.
OWise also has a question list feature that provides suggested questions for you to take into appointments. It can be difficult to remember off the top of your head questions that you have thought about for the weeks or months between appointments. With OWise, you can create a question list specific for a trial and tailored to whether you’re speaking to your research nurse or clinician. Some questions that you may want to ask before taking part in a clinical trial are:
- What is the aim of the trial?
- How will my day-to-day and treatment plan change when being in the trial?
- How long will the trial last?
- How do I find out the results of the trial?
- Do you know the results from the previous phases of the trial?
- Is the data you collect about me kept confidential?
- Who do I contact if I am having any issues?
- Where will I need to travel to take part in the trial?
- Will I be reimbursed for the hospital trips?
- What are the known side effects?
- How may this trial affect my outcome?
For more ideas of questions you should ask before getting involved in a clinical trial, check out Ticking Off Breast Cancer’s post, which was written by Ancora.ai. By having these all documented in your OWise app, you don’t have to worry about keeping them all in your head in the appointment. Once there, you can take notes or use audio recordings to record the answers to each question, which you can review later on to help make your decision.
We hope that this guide has helped in your understanding of clinical trials. If you have any further queries please do not hesitate to get in touch. Clinical trials are not for everyone, and whilst you can receive advice from your doctor, the decision to take part in a trial will be up to you. It’s worth stressing again that if at any point you decide you would no longer like to participate, you have the right to withdraw at any stage.
References
- MetUp UK. (2020). Clinical trials. [online] Available at: https://metupuk.org.uk/research-and-trials/clinical-trials/ [Accessed 1 Dec. 2020]
- Cancer Research UK. (2020). Trial search results. [online] Available at: https://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/clinical-trials-search?populate=Breast%20cancer&f%5B0%5D=field_trial_status%3A4386 [Accessed 1 Dec. 2020]
- Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. (2006). N Engl J Med. 354(8):809‐820.
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. (2005). N Engl J Med. 353(16):1673‐1684.
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. (2005). N Engl J Med. 353(16):1659‐1672.
- Ticking Off Breast Cancer. (2020). Clinical trials: What every cancer patient needs to know [online] Available at: http://www.tickingoffbreastcancer.com/diagnosis/clinical-trials-what-every-cancer-patient-needs-to-know/ [Accessed 1 December 2020]
- Nice.org.uk. (2020). Atezolizumab With Nab-Paclitaxel For Treating PD-L1-Positive, Triple-Negative, Advanced Breast Cancer. [online] Available at: <https://www.nice.org.uk/guidance/ta639/documents/final-appraisal-determination-document> [Accessed 30 November 2020].
- Synexus. (2020). How are clinical trials regulated?. [online] Available at: https://www.synexusclinic.co.uk/faqs/how-are-clinical-studies-regulated/
- Umscheid, C., et al. (2011). Key Concepts of Clinical Trials: A Narrative Review. Postgrad Med. 123(5): 194–204. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3272827/
- Wager, T., Atlas, L.(2018). The neuroscience of placebo effects: connecting context, learning and health. Nat Rev Neurosci. 16(7): 403–418. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6013051/
- Johnston, A. (2020). A Stage IV Breast Cancer-Haver gives her Advice on Clinical Trials. Ancora.ai. [online] Available at: https://www.ancora.ai/posts/a-stage-iv-breast-cancer-haver-gives-advice-on-clinical-trials [Accessed 30 November 2020]
- Nijjar, SK., et al. (2017). Participation in clinical trials improves outcomes in women’s health: a systematic review and Meta-analysis. BJOG. Available at: https://obgyn.onlinelibrary.wiley.com/doi/pdf/10.1111/1471-0528.14528
- Royal College of Physicians. (2018). Recognising research: how research improves patient care. [online]. Available at: https://www.rcplondon.ac.uk/news/recognising-research-how-research-improves-patient-care [Accessed 30 November 2020].
- Chase, M. (2020, Nov). Demystifying clinical trials. Elephants and Tea. [online] Available at: https://elephantsandtea.com/issues/

