Early Stage Hormone Receptor Positive Breast Cancer

It’s time to focus on hormone receptor-positive breast cancer. Specifically, we’re looking at early stages, where cancer has spread in breast tissue and/or to the lymph nodes under the armpit (axillary lymph nodes). Next weeks’ focus will all be on advanced stages, so stay tuned!
Around 75% 1,2,3 of breast cancers are thought to be influenced by oestrogen and progesterone. They are both hormones that travel in the blood to tell other parts of the body how to function. In women, oestrogen and progesterone are the vital sex hormones that regulate menstrual cycles and play an important role in pregnancy.
In breast cancer these hormones have a very different function. Once they have made contact with cancer cells, they stimulate them to grow. This discovery has led to a whole new category of treatment called hormone therapy. Carry on reading to find out what they are and how they work!
For the purpose of the blog, we are solely focusing on hormone receptor positive, HER2 negative breast cancer. If you want to learn more about HER2-positive breast cancer, head to our previous blog.
How common is hormone receptor-positive breast cancer?
Hormone receptor-positive (HR+) breast cancer that is negative for the protein HER2 (see our HER2 blog) is the most common subtype of breast cancer. Cases of HR+ breast cancer increased between the years of 1990 and 2015, specifically in women aged 40 to 69 years. Increased exposure to oestrogen may be one of the reasons for this, caused by earlier age of period onset, higher body mass index and increased use of hormone therapy (such as hormone replacement therapy in postmenopausal women)4.
Did you know? Breast cancer was once referred to as “Nun’s Disease”. A study conducted in 1969 compiled cancer mortality rates in 32,000 Catholic nuns in the USA between 1900 and 1954. They found that nuns had an increased probability of dying from breast, ovarian, and uterine cancer compared with the female population5. This correlation is due to the number of ovulatory menstrual cycles a woman has. Epidemiological studies have found an increased number of cycles increases risk due to the increased exposure to oestrogen. Hence, giving birth, breastfeeding and age of puberty and menopause all influence risk of breast cancer.
It’s important to point out that HR+ breast cancer affects men too. While men have higher levels of male sex hormones (androgens), they also have low levels of circulating oestrogen. 80% of circulating oestrogen is produced through the aromatase pathway (you’ll find out more about that later) and 20% directly by the testes. Up to 90% of breast cancer in men is HR+, and the hormone therapy we will discuss throughout this blog is also given to men6.
How do you know if you’re hormone receptor-positive?
Once you have been diagnosed with breast cancer, a sample of the breast cancer tissue will be analysed during surgery or a biopsy. This will be tested for the presence of oestrogen and progesterone receptors among others. The result that indicates you are HR+ will be given as either a number between 0-8 or a percentage. The higher the score in both cases, the higher prevalence of oestrogen receptors (ER) and/or progesterone receptors (PR). Any score above 1% is characterised as HR+7.
How do oestrogen and progesterone cause cancer growth?
Oestrogen and progesterone bind to receptors in cancer cells that are directly involved in switching genes on and off, changing the cell’s behaviour. When oestrogen binds to its receptor, it turns on genes that tell the cells to keep dividing, driving tumour growth.
The function of progesterone is still under debate. However, recent research has shown that progesterone may actually help to slow down cancer growth8. Once progesterone bound to its receptor, different genes were turned on and off, causing a decrease in the growth of cells. What this means for the future of hormonal treatment is not yet known. 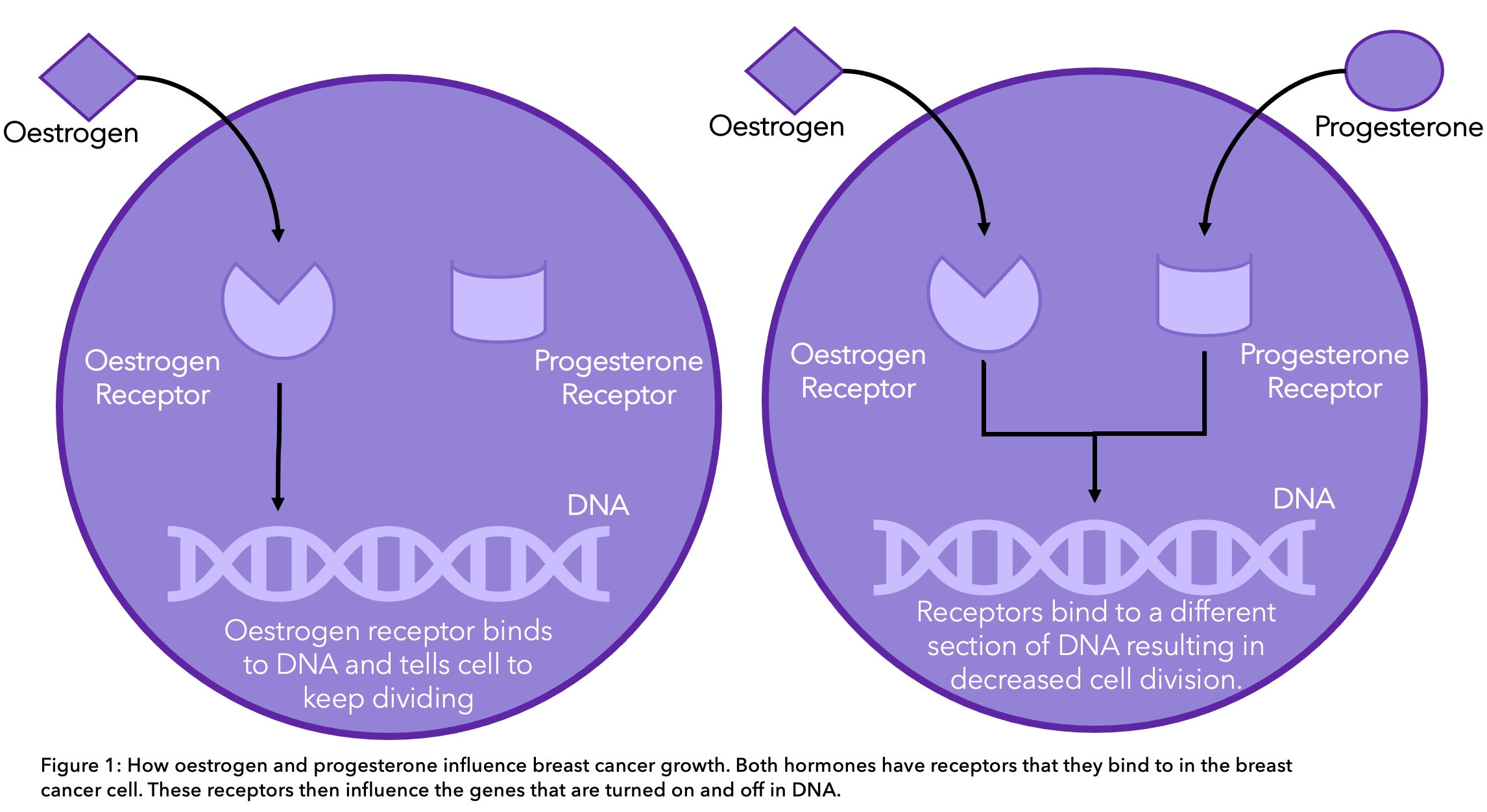
Treatment options
We have pulled together all the different treatment options for early stage HR+ breast cancer that are available on the NHS. We have classified them into two types: hormone therapy and chemotherapy. In this blog, we are going to identify them all and explain how they each work.
Hormone therapy AKA endocrine therapy
Hormone therapy blocks hormones and hormone receptors, suppressing their influence in promoting breast cancer growth. The treatment options available for women depend on whether they are pre- or post-menopausal. This is because oestrogen is produced in different places and therefore treatments need to target different areas.
For premenopausal women, the main source of oestrogen and progesterone are the ovaries. In postmenopausal women, once the menstrual cycle has ceased, the main source of oestrogen is from the aromatase pathway. This is where androgens created in the adrenal glands and ovaries are converted to oestrogen. This happens all over the body, including in the liver, muscle but most significantly in the fat under the skin.
Premenopausal
For premenopausal women and male patients, tamoxifen in the form of an oral tablet is the most commonly prescribed hormone therapy. It works by blocking the oestrogen receptors on the cancer cells. This way, oestrogen is still present in normal amounts in the body, but it cannot activate the cancer cells to grow further (see diagram). 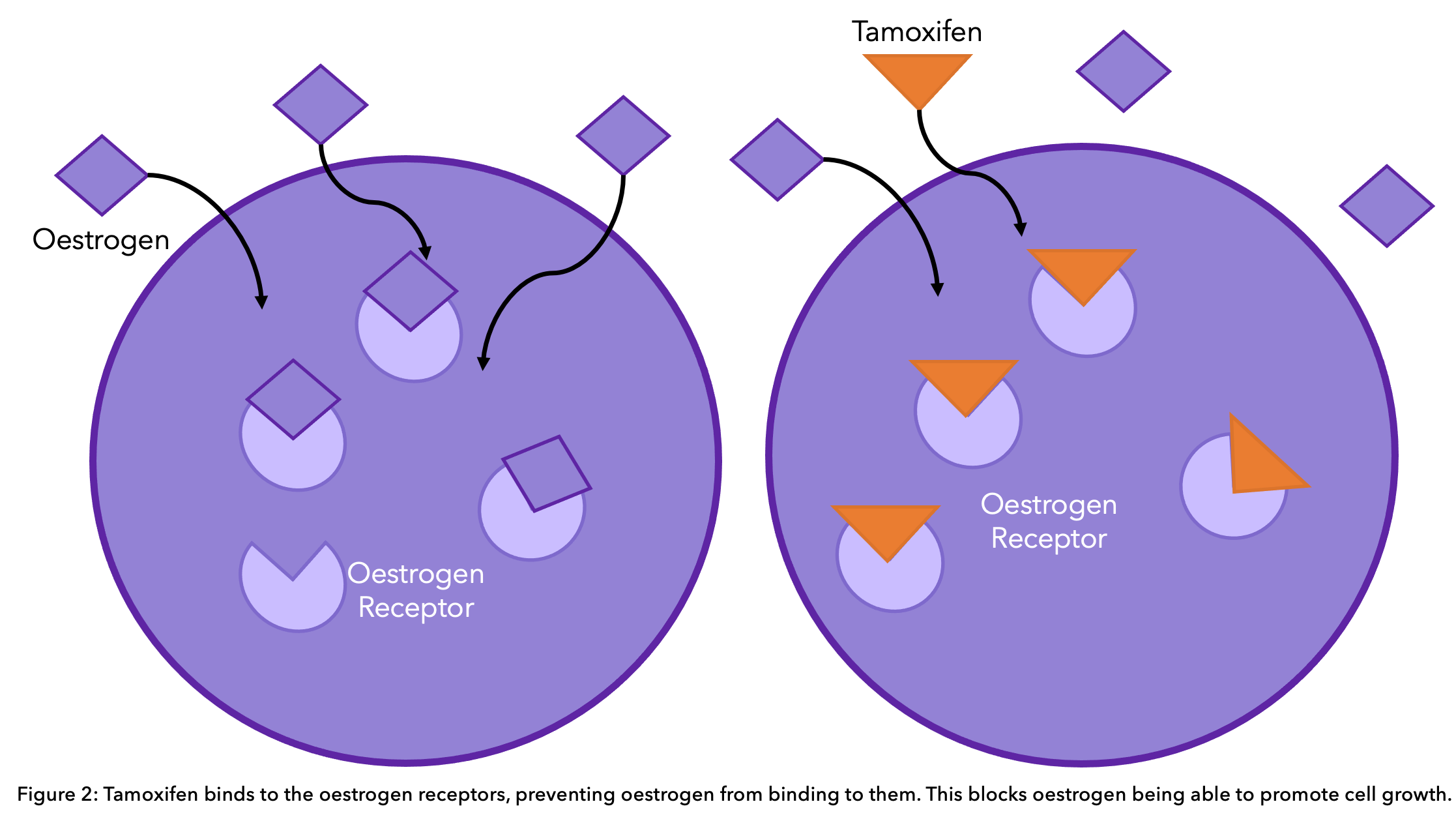
Did you know? Tamoxifen was originally created as a contraceptive pill. Scientists in the 1960’s synthesised the drug hoping it would block oestrogen and act as an effective contraceptive. However, during testing, they found that tamoxifen stimulated, rather than suppressed, ovulation in women9.
Premenopausal women can also be offered treatment that suppresses the function of their ovaries. This therapy is termed ovarian suppression or ovarian ablation. It will be either offered through the removal of the ovaries or treatment that prevents the ovaries from functioning for a limited period of time. Ovarian suppressors block the brain from telling the ovaries how to work. This stops the ovaries from functioning and therefore decreases the presence of oestrogen and progesterone in the body10. The most common ovarian suppressors are11:
- Goserelin (Zoladex®)
- Buserelin (Suprefact®)
- Triptorelin (Decapeptyl®)
- Leuprorelin (Prostap®)
Ovarian suppressors are administered through monthly injections. They can be given by themselves or alongside tamoxifen. The inclusion of ovarian suppressors should be discussed with your doctor and more likely will be offered if there is a high risk of cancer recurrence12. High risk may include people who have lymph-node positive disease (stage N1 or above), tumours that are T2 (2-5 cm across) or greater, and a higher grade, meaning they look significantly different from normal breast cells.
Postmenopausal
Aromatase inhibitors are the most common hormone therapy offered to postmenopasual women. They work by preventing the enzyme “aromatase” from converting other hormones into oestrogen, thus lowering the amount of oestrogen in the body13. These are particularly effective in postmenopausal women due to the aromatase pathway being primarily responsible for oestrogen production. 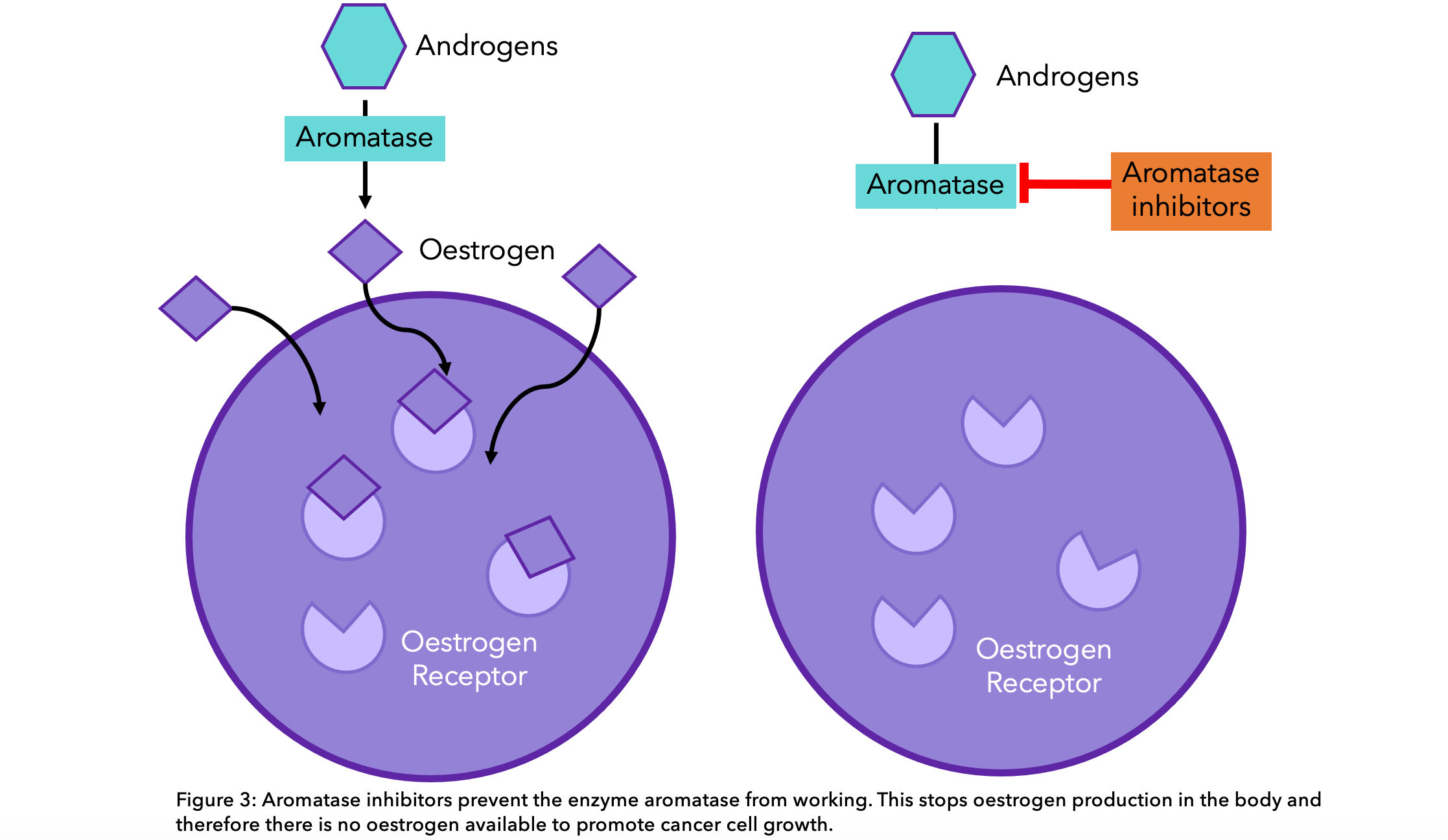 The aromatase inhibitors available in the NHS are13:
The aromatase inhibitors available in the NHS are13:
- Letrozole (Femara®)
- Anastrozole (Arimidex®)
- Exemestane (Aromasin®)
These have been found to decrease circulating oestrogen to undetectable levels 14, thereby reducing tumour growth15. There is no difference in the effectiveness of these three types of medication and the choice of which to have will be between you and your doctor. Postmenopausal women are at high risk of a decrease in bone density (osteoporosis) due to a lack of circulating oestrogens in their body, which can then be accentuated through aromatase inhibitor treatment. Bisphosphonate therapy may be offered to women with early stage breast cancer with cancer present in their lymph nodes. This both decreases the risk of osteoporosis and the spread of breast cancer into the bones12.
Neoadjuvant therapy
Some people with HR+ breast cancer might receive hormone therapy before surgery (this is called neoadjuvant) to reduce the size of the tumour. Hormone therapy has been shown to be as effective as neoadjuvant chemotherapy in postmenopausal women and some premenopausal women. It should be noted that for some premenopausal women neoadjuvant hormone therapy is not as effective at reducing the size of the tumour. The decision between having neoadjuvant hormone therapy or chemotherapy will be decided through a discussion with your doctor12.
How the risk of your cancer coming back will affect your treatment
The risk of cancer coming back can be evaluated through looking at various factors. This includes whether the cancer has spread to your lymph nodes, how large the tumour is, and how different the tumour cells look compared to normal breast cells. The outcome of this evaluation will affect the treatment you will be given. Using genomic profiling tests (see the section below) or the PREDICT tool can also be used to decide the best method of treatment for your cancer.
For postmenopausal women with a low risk of cancer recurrence, for example if there was no cancer in their lymph nodes, then tamoxifen may be offered instead of aromatase inhibitors. This can also be the case if aromatase inhibitors are not tolerated, which is the case for some women. Both treatments significantly reduce rates of recurrence16.
For people with cancer cells in the lymph nodes or a larger, abnormal-looking tumour, extended hormone therapy may be offered. Extended hormone therapy will either be an additional five years of tamoxifen or an aromatase inhibitor. This will mean you could be receiving hormone therapy for 7-10 years. If you are postmenopausal and have been taking tamoxifen for 2-5 years, an aromatase inhibitor would be recommended as this has been shown to have a greater impact in cancer-free survival12. This may also be considered even if you are at low risk of recurrence.
Targeted therapy
The targeted therapy abemaciclib (Verzenio®) in combination with hormone therapy is a treatment option for high risk early HR+, HER2- breast cancer patients. High risk means that the patient either has at least 4 positive lymph nodes, 1-3 positive lymph nodes with grade 3 disease or a tumour size of at least 5cm. Although recurrence in this subtype is uncommon, probability is significantly increased with high risk cancer22. Check out our pathology blog for more information on tumour grade and other markers.
Abemaciclib is a type of cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor that is usually used in advanced HR+ breast cancer. These types of inhibitors are designed to interrupt the growth of cancer cells by targeting CDK4/6 enzymes. The monarchE clinical trial included over 5000 men and women with HR+, HER2- high risk early breast cancer. It was found that after 2 years, 92.2% of patients taking abemaciclib and hormone therapy were alive with no signs of cancer, compared to 88.7% of patients on hormone therapy alone22.
Can I avoid chemotherapy?
In order to decide if chemotherapy can be avoided without affecting overall survival, genomic profiling tests are used in people with early breast cancer that are HR+, HER-negative, and node-negative or have less than 4 lymph nodes containing cancer cells. These tests predict how likely your breast cancer is to come back by analysing the activity of a range of genes in a sample of your breast tissue. The main genomic tests used in the UK are Oncotype DX®17, EndoPredict®18 and Prosigna®19. OncotypeDX® predicts the likelihood of cancer recurrence after surgery, EndoPredict® predicts the probability of cancer coming back within 10 years in people taking hormone therapy for at least five years, and Prosigna® predicts the cancer-free survival in 10 years of ER+ postmenopausal women20. These tests provide a score that indicates the risk of cancer coming back. Usually, the higher the score, the higher the risk. Your specialist will use this score, along with other information about your breast cancer to help decide if chemotherapy would be of benefit to you.
Did you know that the technique that is used to analyse these genes is a quantitative RT-PCR, which is the same test that is currently performed to determine if someone has coronavirus?
Chemotherapy
Chemotherapy is a treatment that uses anti-cancer drugs to target and kill fast-dividing cells in your body. It works by interfering with the cancer cells’ ability to divide and grow. Chemotherapy will be recommended to you after surgery if your cancer is of sufficient risk 12. The risk will depend on the grade and size of your tumour, the number of nodes compromised under your armpit, your age and the score you’ve received in the profiling tests we have mentioned above. In general, the larger the size of the tumour, the higher the grade, number of nodes with cancer and score, the more likely you would benefit from chemotherapy. You may want to enter your cancer characteristics into the PREDICT tool to find out more to what extent chemotherapy can benefit you. Your chemotherapy regimen would include a taxane and an anthracycline drugs:
- Taxane chemotherapy drugs include paclitaxel (Taxol®), nab-paclitaxel (Abraxane®) and docetaxel (Taxotere®).
- Anthracyclines chemotherapy drugs include doxorubicin (Adriamycin®), epirubicin (Ellence®), doxorubicin (Doxil®), daunorubicin (Cerubidine®), mitoxantrone (Novantrone®).
During the COVID-19 pandemic, chemotherapy regimens have slightly changed. Some common intravenous chemotherapy drugs (e.g Taxol®) are being replaced by oral chemotherapy pills such as Capecitabine (Xeloda®), and Abraxane® might be prioritised over Taxol® due to its reduced toxicity (less side effects)21 (if you want to understand the difference between Taxol and Abraxane, head to this Instagram post).
Hormonal therapy side effects
The most common side effects of tamoxifen are hot flushes, nausea, fatigue, bone pain, mood swings, headaches, dry skin, loss of libido, constipation and hair thinning23. Aromatase inhibitors (AIs) side effects also include symptoms of menopause (hot flushes, vaginal dryness and night sweats) and muscle and joint pain. AIs can also lead to osteoporosis (bone thinning) and they may raise cholesterol levels24.
Chemotherapy side effects
Regarding chemotherapy, the side effects can be very diverse as chemotherapy drugs target all fast-dividing cells in your body, and the body has other fast-dividing cells apart from cancer cells such as cells at the hair follicles, the lining of the mouth, skin, nails and intestines. That’s why hair loss, brittle nails and diarrhoea are very common side-effects. Other very common side effects are sickness and vomiting. The chemotherapy drugs anthracyclines can also cause heart function problems. This is why you might have heart tests to check the health of your heart before and during chemotherapy25. Do you know that you can track your side effects with OWise? With over 30 side effects and symptoms to choose from, you can track any changes and share these with your care team and loved ones. Better communication with your care team can make sure you receive the best care possible.
And that’s all the treatments summed up for early hormone receptor-positive breast cancer.
We hope that you now better understand your treatment options and can feel confident in discussions with your care team. Our aim is to make sure you are kept informed so make sure to follow our Instagram and Twitter accounts for any updates.
If you found this article useful and would like to access more personalised information, download OWise today.
References
- Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. 2005. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 123:21–27.
- Lange CA, Yee D. 2008. Progesterone and breast cancer. Womens Health (Lond) 4:151–162.
- Moelans CB, de Ligt J, van der Groep P, Prins P, Besselink N, Hoogstraat M, ter Hoeve N, Lacle M, Kornegoor R, van der Pol C, de Leng W, Barbe E, van der Vegt B, Martens J, Bult P, Smits VT, Koudijs M, Nijman I, Voest E, Selenica P, Weigelt B, Reis-Filho J, van der Wall E, Cuppen E, van Diest PJ. 2019. The molecular genetic make-up of male breast cancer. Endocr Relat Cancer.
- Howlader, N,. et al. 2014. US Incidence Of Breast Cancer Subtypes Defined By Joint Hormone Receptor And HER2 Status. JNCI: Journal Of The National Cancer Institute. 106(5): dju055.
- Britt, K. 2012. The plight of nuns: hazards of nulliparity. The Lancet, 379: 2322-2323.
- Dent, S. F. et al. 2010. Endocrine Therapy For Male Breast Cancer: Rates Of Toxicity And Adherence. Current Oncology, 17(5): 17–21.
- Campbell et al., 2016. The combined endocrine receptor in breast cancer, a novel approach to traditional hormone receptor interpretation and a better discriminator of outcome than ER and PR alone. British Journal of Cancer. 115: 967–973.
- Mohammed, H., Russell, I., Stark, R. et al. 2015. Progesterone receptor modulates ERα action in breast cancer. Nature. 523, 313–317.
- Quirke V. 2017. Tamoxifen from Failed Contraceptive Pill to Best-Selling Breast Cancer Medicine: A Case-Study in Pharmaceutical Innovation. Frontiers in Pharmacology. 8.
- Breast Cancer Now. 2020. Goserelin-zoladex.[online] Available at: https://breastcancernow.org/information-support/facing-breast-cancer/going-through-breast-cancer-treatment/hormone-therapy/goserelin-zoladex [Accessed 12 October 2020].
- Breast Cancer Now. 2020. Ovarian suppression and breast cancer. [online]. Available at: https://breastcancernow.org/information-support/facing-breast-cancer/going-through-breast-cancer-treatment/hormone-therapy/ovarian-suppression-breast-cancer [Accessed 12 October 2020].
- Nice.org.uk. 2020. Early And Locally Advanced Breast Cancer: Diagnosis And Management. [online] Available at: <https://www.nice.org.uk/guidance/ng101/resources/early-and-locally-advanced-breast-cancer-diagnosis-and-management-pdf-66141532913605> [Accessed 12 October 2020].
- Breast Cancer Now, 2019. Aromatase inhibitors (anastrozole, exemestane and letrozole). [online]. Available at: https://breastcancernow.org/information-support/facing-breast-cancer/going-through-breast-cancer-treatment/hormone-therapy/aromatase-inhibitors-anastrozole-exemestane-letrozole [Accessed 12 October 2020].
- Geisler J, Haynes B, Anker G, Dowsett M, Lønning PE. 2002. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized, cross-over study. J Clin Oncol. 20: 751-757.
- Miller WR, Jackson J. 2003. The therapeutic potential of aromatase inhibitors. Expert Opin Investig Drugs.12: 1-12.
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase Inhibitors Versus Tamoxifen In Early Breast Cancer: Patient-Level Meta-Analysis Of The Randomised Trials. 2015. Elsevier BV. Vol 386, no. 10001: 1341-1352.
- Oncotypeiq.com. 2020. About the Oncotype DX Breast Recurrence Score Test. [online] Available at: https://www.oncotypeiq.com/en-US/breast-cancer/healthcare-professionals/oncotype-dx-breast-recurrence-score/about-the-test
- Myriad.com. 2020. EndoPredict executive summary. [online] Available at:https://myriad.com/managed-care/endopredict/
- Cronin M, Sangli C, Liu M, Pho M, Dutta D, Nguyen A. 2007. Analytical validation of the oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clinical Chemistry. 53(6) 1084-1091.
- Nice.org.uk. 2018. Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer. [online] Available at:https://www.nice.org.uk/guidance/dg34/chapter/3-The-diagnostic-tests
- Nice.org.uk. 2020. NHS England interim treatment options during theCOVID-19 pandemic [online]. Available at: https://www.nice.org.uk/guidance/ng161/resources/interim-treatment-change-options-during-the-covid19-pandemic-endorsed-by-nhs-england-pdf-8715724381
- Johnston S, Harbeck N, Hegg R, Toi M, Martin M, Shao Z M , Zhang QY , Martinez Rodriguez JL, Campone M, Hamilton E, Sohn J, Guarneri V, Okada M, Boyle F, Neven P, Cortés J, Huober J, Wardley A, Tolaney SM, Cicin I, Smith IC, Frenzel M, Headley D, Wei R, San A, Belen and Hulstijn M, Cox J, O’Shaughnessy J, Rastogi P. (2020). Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2−, Node-Positive, High-Risk, Early Breast Cancer (monarchE). Journal of Clinical Oncology.
- Breastcancer.org. 2020. Tamoxifen (Brand names: Novaldex, Soltamox). [online]. Available at: https://www.breastcancer.org/treatment/hormonal/serms/tamoxifen
- Cancer.org. 2020. Aromatase Inhibitors for Lowering beast cancer risk. [online]. Available at: https://www.cancer.org/cancer/breast-cancer/risk-and-prevention/aromatase-inhibitors-for-lowering-breast-cancer-risk.html#:~:text=What%20are%20the%20risks%20and,to%20stop%20taking%20the%20drugs.
- Cancerresearchuk.org. 2020. Doxorubicin [online]. Available at: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatment/cancer-drugs/drugs/doxorubicin [Accessed 12 October 2020]