
A breast cancer diagnosis can be a shock for young women as it comes with a number of unique challenges including the ability to have children after treatment. Women who are diagnosed with breast cancer before menopause make up 1 in 4 of the overall breast cancer cases1. Both the cancer treatment themselves (such as chemotherapy or hormone therapy) and the time scales of these treatments make young women feel overwhelmed. They need to make decisions regarding their reproductive wishes very quickly in order to treat their cancer as soon as possible. In this blog, we outline research on the known impacts of breast cancer treatments on current and future fertility, what fertility preservation options are available and their practical considerations and effects.
“24hrs after my diagnosis I had a breast nurse at my house going through what to expect- surgery, what will happen, recovery time, type of chemo I’ll have, course of radiotherapy to ‘cover all areas’ and be safe… I was also asked there and then if I wanted children. As a single woman with no kids I had the option to freeze eggs before chemo to use if and when I met someone in the future. Whoa… Yesterday I was told I had cancer and now I HAVE to make a decision on if I want kids…” -Leanne, breast cancer survivor who had two beautiful children after cancer through IVF and egg freezing
Under 45 breast cancer: What is the data?
Every year, in the UK, around 5000 women under the age of 45 are diagnosed with breast cancer.2 Furthermore, in 2020, 89,500 new breast cancers in women between 15-39 years old were diagnosed within the US.3 Breast cancer in this age range is usually referred to as Adolescent and Young Adult Breast Cancer (AYA), and it is sadly the most common type of cancer affecting adolescent and young adult women. 4
Young women are more likely than older women to have fast-growing subtypes of breast cancer5:
- 49% are oestrogen receptor-positive;
- 22.8% triple-negative (hormone receptors negative and HER2-negative);
- 17.9% triple positive (hormone receptors positive and HER2-positive);
- 10.3% HER2-positive
Did you know that with the OWise app, you can save all of your breast cancer treatments in one single place? Also, the app generates a list of suggested questions based on your breast cancer subtype … “I enjoyed using the app as it was ideal to collate all information and appointments on my treatment. Being able to collate all treatment details makes one less thing to worry about, so very important as you receive numerous letters, appointments each week” (Michelle, OWise user).
What are the Impacts of Breast Cancer Treatments on Current and Future Fertility?
Most of the life saving breast cancer treatments such as chemotherapy and hormone therapies can affect fertility adversely. This section covers how these treatments affect the ovarian function and therefore fertility.
Fertility issues rank second in concerns that younger women diagnosed with breast cancer7 8
Chemotherapy: Impact on fertility
Under half of young women with breast cancer are treated with chemotherapy 9. Some women lose their periods (amenorrhea) as soon as they start chemotherapy, whilst others continue to menstruate during treatment. For the first group, even a year after treatment has finished, there is only a small proportion that restart their periods. Previous studies have reported that periods can take from 3-4 months to 2 years to begin again. However, if the ovarian reserve is completely depleted, a woman will no longer menstruate 8.
Did you know that all women are born with a fixed number of eggs? The ovarian reserve is a term that refers to the number and quality of your eggs. These parameters determine the capacity of the ovary to provide egg cells that are capable of fertilisation resulting in a healthy and successful pregnancy.
It is important to note the effects on fertility depend on the type of chemotherapy, the dosage and the ovarian reserve. For example, some therapies, such as cyclophosphamide (Cytoxan®, Neosar®), can be extremely harsh to your ovaries, while others, such as 5-fluorouracil have little effect on the ovarian reserve. 10 11
There are two tests typically used by doctors to check ovarian reserve: the concentration of anti-Mullerian hormone (AMH) and the ultrasonographic antral follicle count.12

Hormone Therapy: Impact on Fertility
There is little data demonstrating the direct effect of tamoxifen on fertility. A recent study with young breast cancer survivors on tamoxifen found they did not have a decreased ovarian reserve compared to those not taking tamoxifen.13
Ovarian suppression therapy with pharmacological injection such as goserelin (Zoladex®) is another treatment option for young women with estrogen receptor positive breast cancer that will stop the production of oestrogen and stop menstruation temporarily. However, a 2018 study found that young women with early stage hormone receptor negative breast cancer that were treated with Zoladex® in addition to chemotherapy (including cyclophosphamide) before surgery were much less likely to be infertile after treatment.14 To learn more about treatment before surgery, check out our blog in neoadjuvant therapy in breast cancer.
Fertility preservation: What are the options?
- Embryo Freezing (Embryo cryopreservation) & Egg Freezing (Oocyte cryopreservation)
These are the most well-established methods of fertility preservation15. The main difference is that embryo freezing requires sperm to fertilize the egg before freezing. The process of embryo and egg freezing are basically the same, although the timings can differ. For embryo freezing, timing in terms of menstrual cycle is more important than for egg freezing, where the timing of egg collection is less important.
Although these are well-established methods of fertility preservation, they are not 100% effective. The success rate of both embryo and egg freezing depends on your age and the amount of quality eggs removed after stimulation 8. The egg or embryo may be damaged during thawing or may not implant into your uterus correctly.
“I did get IVF counselling during my IVF process and it gave me a good understanding of how to explain to Thea when she is older how she came about and how I tried so hard for so long to get her. And now Hudson is here it feels complete now.” –Leanne, breast cancer survivor
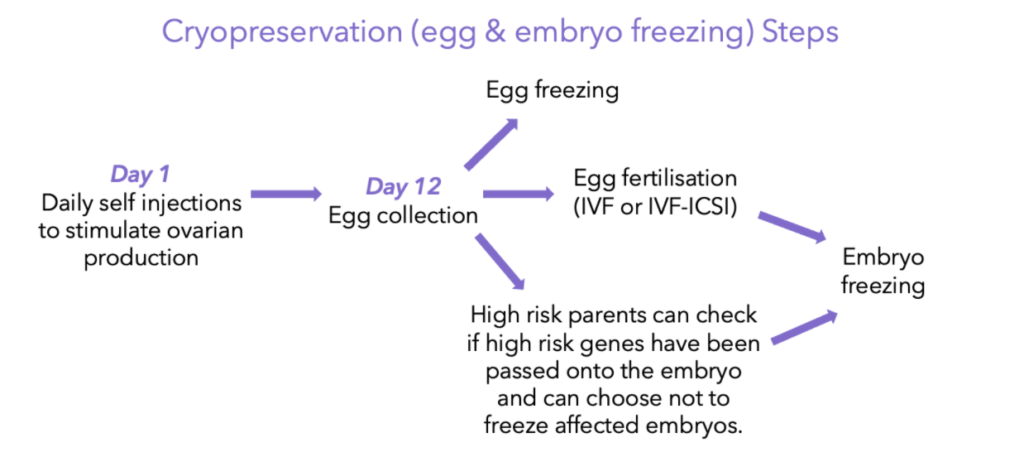
Steps for embryo & egg freezing:
- Ovaries are stimulated with a hormone treatment, called gonadotropic hormones, in order to induce your body to release as many eggs as possible. This usually takes 10-12 days of daily gonadotropin injections8. The development of the eggs are closely monitored with ultrasounds and hormone testing, but most women can carry on with their daily routines.
- Once the ovaries have been stimulated and release a certain amount of mature eggs, the mature eggs are removed. During this procedure you are conscious but sedated. In total the stimulation and retrieval process can take 12-14 days, and as tumour staging and other various diagnostic tests needed before treatment take a similar 12-14 day time-frame to be completed, the egg retrieval process should not delay start in treatment.8
- For egg freezing: this is an option for people who don’t have a partner or don’t want to use a sperm donor to fertilise their egg. The eggs are frozen and stored, in a process frequently referred to as “egg banking”. Later on, when the time has come and you do want to become pregnant, the egg is thawed, fertilised with sperm and implanted into your uterus.
- For embryo freezing: once the egg is removed, it is fertilised with sperm in a lab dish for it to form into an embryo. This is known as “IVF” or in vitro fertilisation. If the egg is fertilised, it is then frozen to preserve it for when you are ready to undergo pregnancy after cancer treatment.
An important note on breast cancer & ovarian stimulation
Scientists used to believe that it was necessary for ovary stimulation to occur at the beginning of a women’s menstrual cycle. This caused issues for breast cancer patients as it potentially meant having to wait a month before commencing an embryo or egg freezing process and pushing back cancer treatments. However, recent research has shown that it is not necessary to wait until the start of the menstrual cycle to begin ovarian stimulation for embryo/egg freezing. Scientists realised that eggs can be released at any point during a menstrual cycle with the stimulation of gonadotropins,16 and therefore the ovarian stimulation can be started at any point. This opened up more fertility preservation options for young women diagnosed with breast cancer who cannot wait a month to commence treatment.
Ovarian stimulation versus controlled ovarian stimulation
In theory, inducing more hormones during ovarian stimulation could stimulate hormone receptor positive breast cancer cells. However, the research supports that fertility preservation methods such as embryo and egg cryopreservation and ovarian stimulation are safe for breast cancer patients17 18. A long term study over 23 years of 425 women with breast cancer who underwent fertility preservation processes found that both fertility treatments and pregnancy did not have an effect on survival or cancer recurrence18 19. This is an important study, as unlike many other research studies it studied the long-term effects of fertility preservation and pregnancy on cancer recurrence and outcomes.
Although current studies indicate that ovarian stimulation as part of fertility treatment is a safe option for breast cancer patients, controlled ovarian stimulation is an option which can help reduce circulating oestrogen levels during ovarian stimulation8 18. Controlled ovarian stimulation involves taking tamoxifen or an aromatase inhibitor before and after ovarian stimulation10 15 20. This lowers the amount of circulating oestrogen without affecting fertilisation or cancer recurrence.15
- Ovarian Tissue Freezing (Ovarian tissue cryopreservation)
This method is currently still being established, however it has recently been reclassified as non experimental by the American Society of Reproductive Medicine and there are promising results so far21 22. It aims to preserve ovarian function despite the damaging effects of cancer treatments. It involves a small surgical procedure (that usually does not require a hospital stay) where all or part of your ovarian tissue is removed. This tissue is molecularly tested to ensure that it contains no cancer cells, and then is preserved by being frozen (cryopreserved)8. It is later thawed and re-transplanted once you finish your cancer treatment course. Ovarian tissue typically takes 4-5 months to become hormonally active again once it is transplanted 8. Afterwards eggs can be stimulated and fertilised in a lab.
So far, more than 60 live births following ovarian tissue freezing have been recorded23. It is important to note that this is not an option if there is a high risk of ovarian metastases (breast cancer spreading to the ovaries) or if there is a high risk of ovarian cancer.8
- Ovarian Function Suppression: Using gonadotropin releasing hormone agonists
This is not a fertility preservation method on its own, but it is sometimes used alongside chemotherapy in order to reduce chemotherapy’s toxicity on the ovaries. Ovarian function suppression involves using a gonadotropin releasing hormone (GnRH) agonist (a monthly injection with goserelin (Zoladex®)) in order to stop the ovaries from ovulating and stop menstruation. In other words, it temporarily stops ovarian function. The exact mechanism by which it does this is not fully known as of yet 10 15 but the idea is to temporarily stop ovarian function to protect it from the effects of chemotherapy. The effectiveness of this method remains controversial 10 15 24. Several studies have shown that it can help preserve fertility and prevent ovarian failure after treatment 15 24 25. A 2018 study of 873 breast cancer patients who were randomly chosen to either undergo ovarian suppression or not found no difference in overall survival between the groups regardless of whether the breast cancer was hormone receptor positive or not24. This indicates ovarian suppression is safe for breast cancer patients. Moreover, the study found that ovarian suppression significantly reduced the risk of premature ovarian insufficiency (POI) and was associated with a higher future pregnancy rate24. These results indicate the potential of this fertility preservation method for women with breast cancer.
Reproductive counselling and useful services
It is part of NICE guidelines in the UK that breast cancer patients should be promptly referred to a fertility specialist, regardless of whether they have a partner, in order to discuss fertility preservation options before they start any treatment26. If you are diagnosed with breast cancer and having future children is important to you, you should be referred to a fertility specialist or counsellor. Fertility issues may not be top on a doctor’s agenda, so if you are not asked about this be sure to bring it up yourself as it can be a time-sensitive matter. Breast Cancer Now provides a good outline of the initial steps of this process, and a list of suggested questions to ask your treatment team. Your fertility clinic should also give you access to a counsellor before, during or after IVF treatment regardless of the outcome 27.
We hope that you now better understand your treatment options and their impact on fertility and can feel confident in discussions with your care team. At OWise, we want to make sure you are kept informed, check out the suggested list of questions and personalised treatment reports that the app generates for you to feel empowered to discuss your options in your next consultation with your doctors. Follow our Instagram and Twitter for any updates. Any questions? Get in touch
Useful Links
- https://breastcancernow.org/information-support/facing-breast-cancer/breast-cancer-in-younger-women
- https://www.nccn.org/patients/guidelines/content/PDF/aya-patient.pdf
References:
- Mailliez, Audrey et al. “Chimiothérapie adjuvante de cancer du sein et fertilité: estimation de l’impact, options de préservation et place de l’oncologue” [Adjuvant chemotherapy for breast cancer and fertility: estimation of the impact, options of preservation and role of the oncologist]. Bulletin du cancer vol. 98,7 (2011): 741-51. doi:10.1684/bdc.2011.1391
- Younger Women with Breast Cancer. breastcancernow.org/sites/default/files/publications/pdf/bcc66_younger_women_with_breast_cancer_web.pdf.
- Miller, Anthony B. “Final Results of the UK Age Trial on Breast Cancer Screening Age.” The Lancet Oncology, vol. 21, no. 9, 2020, pp. 1125–1126., doi:10.1016/s1470-2045(20)30428-9.
- Cathcart-Rake et al., “Breast Cancer in Adolescent and Young Adult Women Under the Age of 40 Years”JCO Oncology Practice 17, no. 6 (June 01, 2021) 305-313.
- Keegan, Theresa H M et al. “Occurrence of breast cancer subtypes in adolescent and young adult women.” Breast cancer research : BCR vol. 14,2 R55. 27 Mar. 2012, doi:10.1186/bcr3156
- Lallo, Fiona, et al. “BRCA1, BRCA2 And TP53 Mutations in Very Early-Onset Breast Cancer with Associated Risks to Relatives.” European Journal of Cancer, vol. 42, no. 8, 2006, pp. 1143–1150., doi:10.1016/j.ejca.2005.11.032.
- Gorman, Jessica R et al. “Young breast cancer survivors: their perspectives on treatment decisions and fertility concerns.” Cancer nursing vol. 34,1 (2011): 32-40. doi:10.1097/NCC.0b013e3181e4528d
- Warner, Ellen et al. “Update on fertility preservation for younger women with breast cancer.” CMAJ : Canadian Medical Association journal = journal de l’Association medicale canadienne vol. 192,35 (2020): E1003-E1009. doi:10.1503/cmaj.200245
- Partridge, Ann H et al. “Web-based survey of fertility issues in young women with breast cancer.” Journal of clinical oncology : official journal of the American Society of Clinical Oncology vol. 22,20 (2004): 4174-83. doi:10.1200/JCO.2004.01.159
- Sonmezer, Murat, and Kutluk Oktay. “Fertility Preservation in Young Women Undergoing Breast Cancer Therapy.” The Oncologist, vol. 11, no. 5, 2006, pp. 422–434., doi:10.1634/theoncologist.11-5-422.
- de Pedro, María et al. “Fertility preservation and breast cancer: a review.” Ecancermedicalscience vol. 9 503. 3 Feb. 2015, doi:10.3332/ecancer.2015.503
- Hansen, Karl R., et al. “Correlation of Ovarian Reserve Tests with Histologically Determined Primordial Follicle Number.” Fertility and Sterility, vol. 95, no. 1, 2011, pp. 170–175., doi:10.1016/j.fertnstert.2010.04.006.
- Shandley, Lisa M et al. “Impact of tamoxifen therapy on fertility in breast cancer survivors.” Fertility and sterility vol. 107,1 (2017): 243-252.e5. doi:10.1016/j.fertnstert.2016.10.020
- Halle C F Mooreet al., Final Analysis of the Prevention of Early Menopause Study (POEMS)/SWOG Intergroup S0230, JNCI: Journal of the National Cancer Institute, Volume 111, Issue 2, February 2019, Pages 210–213, https://doi.org/10.1093/jnci/djy185
- Kim, Hoon et al. “Fertility preservation for patients with breast cancer: The Korean Society for Fertility Preservation clinical guidelines.” Clinical and experimental reproductive medicine vol. 44,4 (2017): 181-186. doi:10.5653/cerm.2017.44.4.181
-
von Wolff, Michael et al. “Ovarian stimulation to cryopreserve fertilized oocytes in cancer patients can be started in the luteal phase.” Fertility and sterility vol. 92,4 (2009): 1360-1365. doi:10.1016/j.fertnstert.2008.08.011
- Goldrat, Oranite, et al. “Pregnancy Following Breast Cancer Using Assisted Reproduction and Its Effect on Long-Term Outcome.” European Journal of Cancer, vol. 51, no. 12, 2015, pp. 1490–1496., doi:10.1016/j.ejca.2015.05.007.
- Letourneau, Joseph M et al. “Fertility preservation before breast cancer treatment appears unlikely to affect disease-free survival at a median follow-up of 43 months after fertility-preservation consultation.” Cancer vol. 126,3 (2020): 487-495. doi:10.1002/cncr.32546
- Marklund, Anna et al. “Reproductive Outcomes After Breast Cancer in Women With vs Without Fertility Preservation.” JAMA oncology vol. 7,1 (2021): 86-91. doi:10.1001/jamaoncol.2020.5957
- Oktay, Kutluk et al. “Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation.” Journal of clinical oncology : official journal of the American Society of Clinical Oncology vol. 23,19 (2005): 4347-53. doi:10.1200/JCO.2005.05.037
- Practice Committee of the American Society for Reproductive Medicine. Electronic address: asrm@asrm.org. “Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion.” Fertility and sterility vol. 112,6 (2019): 1022-1033. doi:10.1016/j.fertnstert.2019.09.013
- Pacheco, Fernanda, and Kutluk Oktay. “Current Success and Efficiency of Autologous Ovarian Transplantation: A Meta-Analysis.” Reproductive sciences (Thousand Oaks, Calif.) vol. 24,8 (2017): 1111-1120. doi:10.1177/1933719117702251
- Donnez, Jacques, and Marie-Madeleine Dolmans. “Ovarian cortex transplantation: 60 reported live births brings the success and worldwide expansion of the technique towards routine clinical practice.” Journal of assisted reproduction and genetics vol. 32,8 (2015): 1167-70. doi:10.1007/s10815-015-0544-9
- Lambertini, Matteo et al. “Gonadotropin-Releasing Hormone Agonists During Chemotherapy for Preservation of Ovarian Function and Fertility in Premenopausal Patients With Early Breast Cancer: A Systematic Review and Meta-Analysis of Individual Patient-Level Data.” Journal of clinical oncology : official journal of the American Society of Clinical Oncology vol. 36,19 (2018): 1981-1990. doi:10.1200/JCO.2018.78.0858
- Blumenfeld Z, Zur H, Dann EJ. Gonadotropin-Releasing Hormone Agonist Cotreatment During Chemotherapy May Increase Pregnancy Rate in Survivors. The Oncologist. 2015 Nov;20(11):1283-1289. DOI: 10.1634/theoncologist.2015-0223.
- Addressing Fertility Issues in NICE’s Breast Cancer Guidance. breastcancernow.org/sites/default/files/files/addressing_fertility_issues_in_nices_breast_cancer_guidance_-_breast_ca.pdf.
- “In Vitro Fertilisation: Information for the Public: Fertility Problems: Assessment and Treatment: Guidance.” NICE, www.nice.org.uk/Guidance/CG156/IFP/chapter/in-vitro-fertilisation.
