 Immunotherapy is an emerging way to treat breast cancer. Instead of directly targeting cancer cells, immunotherapy works by boosting the immune system to recognise and destroy them1. This blog will explain how the immune system works, the different types of immunotherapy drugs, and the pros and cons of using this approach.
Immunotherapy is an emerging way to treat breast cancer. Instead of directly targeting cancer cells, immunotherapy works by boosting the immune system to recognise and destroy them1. This blog will explain how the immune system works, the different types of immunotherapy drugs, and the pros and cons of using this approach.
“When I found out I qualified for an immunotherapy drug that had just been approved for early stage triple negative breast cancer I was over the moon. I’m so grateful to pembrolizumab (Keytruda®). It means my chances of recurrence are massively reduced. It is a very exciting drug for triple negative breast cancer and will hopefully lead to a lot more people surviving this aggressive type of breast cancer.” -Carly Moosah
Understanding the immune system
It is important to understand the immune system before trying to understand immunotherapy. The immune system is our body’s natural defence against pathogens like bacteria, viruses, fungi, parasites and abnormal cells. It is made up of a network of proteins, cells, tissues, and organs working together. It can tell the difference between things that are ‘self’ and ‘non-self’. This stops it from attacking essential cells and proteins, while ‘non-self’ entities, such as pathogens and abnormal cells, can be destroyed. The immune system can destroy pathogens and abnormal cells in two ways: innate immunity and adaptive immunity.
Innate immunity
Innate immunity is the body’s first-line response to diseases and foreign invaders,3. It reacts quickly whenever the immune system recognises something as ‘non-self’ or foreign. This is sometimes called the non-specific immune response3. This quick reaction is key in preventing the spread of pathogens throughout the body.
Several systems work together in this defence, including physical barriers like the skin, eyelashes and mucous. Enzymes and certain immune cells can also break down foreign molecules directly.
Adaptive immunity
Adaptive immunity, also called acquired immunity, is a specialised defence mechanism that specifically targets and destroys foreign invaders3. It develops over time and recognises and remembers specific proteins, known as antigens, on the surface of foreign invaders. These antigens help the immune system to distinguish between ‘self’ and ‘non-self’. The memory of the immune system is a central to adaptive immunity, which explains why you may only get an illness once in your life and serves as the foundation of many vaccines3.
The adaptive system is made up of T-cells, B-cells, and antibodies:
- T-cells, or T-lymphocytes, are white blood cells that can directly attach to and kill pathogens (killer t-cells), or signal to other immune cells to fight an infection (helper t-cells) 3,4.
- B-cells, or B-lymphocytes, are white blood cells that are activated by helper t-cells. This allows them to multiply and turn into plasma cells, which can produce large amounts of highly specific antibodies.
- Antibodies are able to attach to the antigens on the surface of foreign invaders, signalling to other cells to destroy and break-down the pathogen3.
How can cancer hide from the immune system?
Cancer is when cells grow abnormally in the body, which can lead to cells dividing uncontrollably and forming tumours
There are times when cells in our body may be abnormal or become damaged, and in many cases our immune system is able to identify these as ‘non-self’ and destroy them. In the early stages, the immune system is able to target and destroy cancer cells5,6. However, cancer cells can grow rapidly and change (mutate) quicker than the immune system can keep up with, making it challenging for the immune system to destroy them5. Additionally, cancer cells can actively hide from the immune system by changing their surface antigens or creating conditions around the tumour (the microenvrionment) supportive for cancer growth5.
How does immunotherapy work?
Immunotherapy treatments boost the bodies natural immune response to cancer cells, helping it to recognise and attack them more effectively. It does this even when the cancer cells have developed the ability to hide from the immune system. There are a range of immunotherapy drugs that each work in a different way. They can be divided into active and passive immunotherapy:
- Active immunotherapy –Provides a long-term benefit even after treatment has finished
- Passive immunotherapy –Provides a benefit for as long as it is being given
Immunotherapies that are approved or are being trialled in breast cancer include7,8:
- Immune checkpoint inhibitors
- Monoclonal antibodies
- Antibody-drug conjugates
- Adoptive cell therapies / CAR-T-cell therapy
- Cancer vaccines
Active immunotherapy
Active immunotherapy can improve the immune system’s ability to recognise and destroy cancer cells in the long term. Active immunotherapies that are approved or under investigation for breast cancer include immune checkpoint inhibitors, adoptive cell therapies (e.g. CAR-T-cell therapy) and cancer vaccines7,8.
Sometimes immunotherapy can belong to more than one treatment type as they can work in more than one way. For example, pembrolizumab (Keytruda®), a drug approved to treat triple negative breast cancer (TNBC), is considered a monoclonal antibody and an immune checkpoint inhibitor.
Immune checkpoint inhibitors:
Immune checkpoints are proteins, such as PD-L1, on the surface of cells that help the immune system to recognise them as ‘self’. This happens through these immune checkpoint proteins binding to their partner proteins. In PD-L1’s case, its partner protein is PD-1 which is found on the surface of T-cells. When PD-L1 binds to PD-1 the T-cell recognises the immune checkpoint protein as ‘self’ and does not attack it.
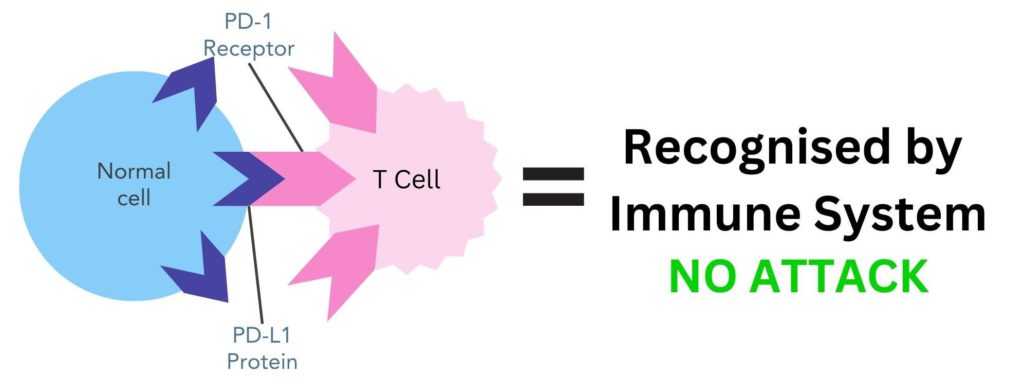 Figure 1. The PD-L1 protein on the surface of normal cells binds to its partner protein, PD-1, on the surface of T-cells. This makes the immune system recognise this cell as ‘self’ and does not attack it.
Figure 1. The PD-L1 protein on the surface of normal cells binds to its partner protein, PD-1, on the surface of T-cells. This makes the immune system recognise this cell as ‘self’ and does not attack it.
Cancer cells can use checkpoint inhibitor proteins to avoid being detected by the immune system. For example, cancer cells can have PD-L1 proteins on the surface of their cells, enabling them to bind to PD-1 partner proteins on T-cells 9. This tricks the body into thinking they are ‘self’, therefore the body does not destroy them.
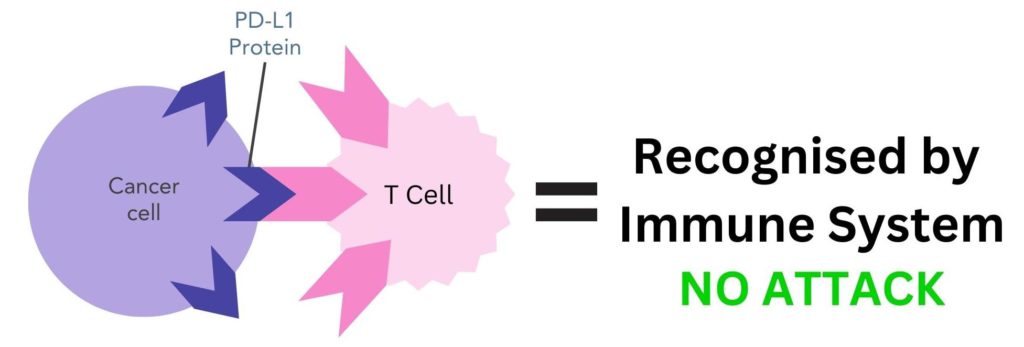 Figure 2. Cancer cells can have PD-L1 proteins on the surface that bind to PD-1 proteins on T-cells. This makes the immune system recognise this cell as ‘self’ and does not attack it.
Figure 2. Cancer cells can have PD-L1 proteins on the surface that bind to PD-1 proteins on T-cells. This makes the immune system recognise this cell as ‘self’ and does not attack it.
Immune checkpoint inhibitors approved to treat breast cancer aim to stop the binding of PD-L1 on cancer cells to PD-1 on T-cells, which then allows the immune system to recognise and destroy them. There are two immune checkpoint inhibitors currently approved to treat breast cancer: pembrolizumab and atezolizumab.
Pembrolizumab:
- Pembrolizumab (Keytruda®) is a monoclonal antibody that is also a immune checkpoint inhibitor
- It is approved to treat primary and secondary triple negative breast cancer (TNBC)
- It is currently being trialled in primary hormone receptor-positive (HR+) breast cancer
- It blocks the PD-1 protein on T-cells, preventing the PD-L1 on cancer cells from binding
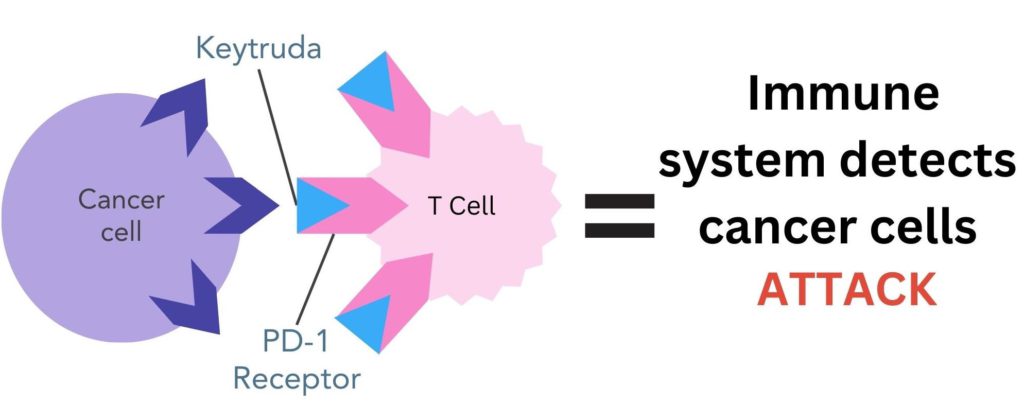 Figure 3. Pembrolizumab (Keytruda®) binds to PD-1 proteins on the surface of T-cells. This stops the PD-L1 protein on the surface of cancer cells from binding to PD-1, enabling the immune system to recognise cancer cells as ‘non-self’ and destroy them.
Figure 3. Pembrolizumab (Keytruda®) binds to PD-1 proteins on the surface of T-cells. This stops the PD-L1 protein on the surface of cancer cells from binding to PD-1, enabling the immune system to recognise cancer cells as ‘non-self’ and destroy them.
Want to read about real patient experiences on pembrolizumab for secondary breast cancer? Read ‘Keytruda for Secondary Breast Cancer: Science and Stories‘.
Atezolizumab:
- Atezolizumab (Tecentriq®) is a monoclonal antibody that is also an immune checkpoint inhibitor
- It is approved to treat secondary TNBC
- It is currently being trialled in primary TNBC
- It blocks the PD-L1 proteins on cancer cells, preventing them binding to PD-1 proteins on T-cells
- To receive treatment cancer cells need to have a certain amount of the PD-L1 protein on cancer cells for it to be effective
T-cell therapy:
Adoptive cell therapy is when immune cells (T-cells) are removed from the body, grown in the lab, and put back into the body using an intravenous (IV) drip. The aim is to activate or modify immune cells to improve the immune response against cancer cells. There are 3 main types of adoptive cell therapy:
- Tumour infiltrating lymphocyte (TIL)-based therapy
- T-cell receptor (TCR) gene therapy
- CAR T-cell (CAR-T) therapy
CAR-T therapy has shown to be the most promising in clinical trials. CAR-T therapy is when T-cells are collected and genetically altered in the lab to enable them to detect and target cancer specific antigens more effectively. The altered cells are grown in the lab and then reintroduced into the bloodstream via IV drip.
CAR-T therapy is approved to treat some types of blood cancers, but has not yet been approved to treat breast cancer. However in 2022, there were 22 ongoing clinical trials for CAR-T therapy in breast cancer. Further research is needed before CAR-T therapy can be done in breast cancer patients10.
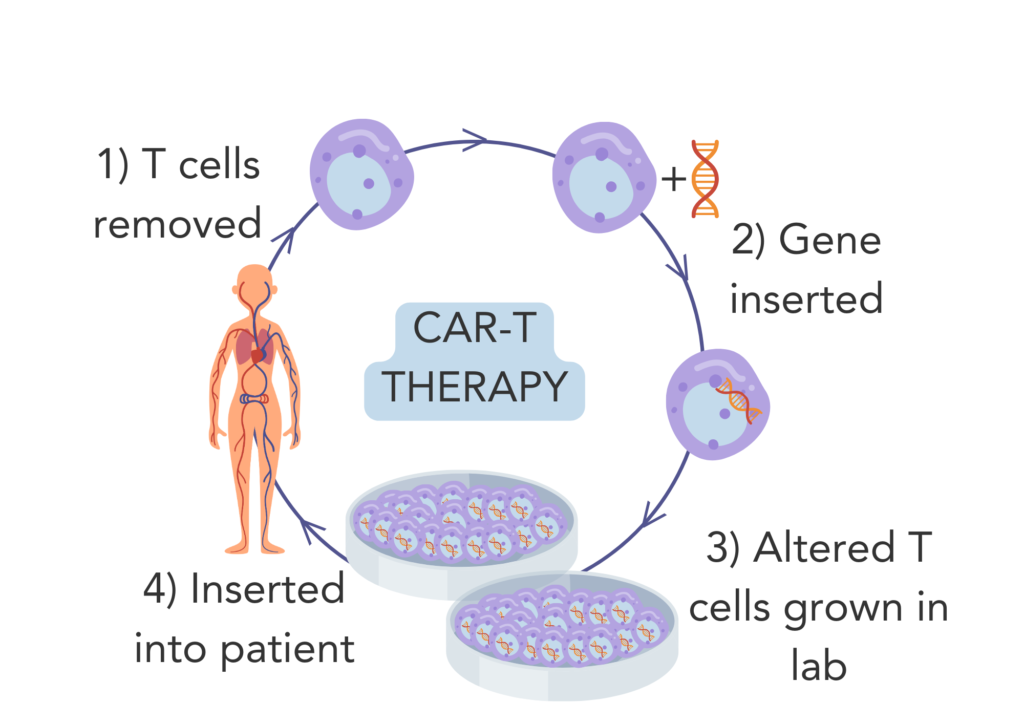 Figure 4. CAR-T therapy 1) T-cells are removed from the patient. 2) T cells are genetically altered in the lab. 3) The genetically altered T-cells are grown in the lab. 4) The genetically altered T-cells are reinserted into the patient.
Figure 4. CAR-T therapy 1) T-cells are removed from the patient. 2) T cells are genetically altered in the lab. 3) The genetically altered T-cells are grown in the lab. 4) The genetically altered T-cells are reinserted into the patient.
Cancer vaccines:
Vaccines work by stimulating the body’s immune system to recognise and defend against specific pathogens, such as viruses or bacteria. They do this through introducing antigens into the body, initiating an immune response. This is helpful if this pathogen is encountered in the future as the immune system will remember it (adaptive immunity), leading to the pathogen being quickly recognised and destroyed
Cancer vaccines work in a similar way, they include certain cancer-specific antigens to enable the immune system to recognise and destroy cancer cells with these antigens on the cell surface11. Cancer vaccines can be tailored and personalised to an individuals specific cancer antigens, or they can be more generalised, targeting common antigens of cancer cells.
Sipuleucel-T (Provenge®) is currently the only cancer vaccine approved. It was approved by the FDA in 2010 to treat people with secondary prostate cancer11,12. Clinical trials for vaccines in breast cancer patients are ongoing. The NeuVax vaccine is considered one of the most advanced vaccines for breast cancer under investigation13. It is being developed for HER2-positive (HER2+) breast cancer and has merged a protein similar to HER2 with the drug granulocyte and macrophage colony stimulating factor (GM-CSF). GM-CSF can stimulate the immune system, therefore the aim is to have this vaccine after standard treatment to stimulate the immune system to recognise and destroy remaining HER2+ cancer cells.
Passive immunotherapy
Unlike active immunotherapy, passive immunotherapy provides a benefit for as long as the treatment is being given, but not after it has finished. These include monoclonal antibodies, such as trastuzumab (Herceptin®), and antibody-drug conjugates, such as trastuzumab deruxtecan (Enhertu®).
Monoclonal antibodies:
Monoclonal antibodies (mAbs) are lab-made antibodies that have been designed to target specific antigens on the surface of cells, such as cancer cells14,15. Monoclonal antibodies bind specifically to the target antigen, forming an antigen-antibody complex. When monoclonal antibodies bind the breast cancer cells this changes the behaviour of the cell, often leading to the destruction of the cell.
Different monoclonal antibodies can work in different ways. Some are targeted therapies, meaning they have a specific target on cancer cells that they can bind to and destroy16. Alternatively, others act like immunotherapy, as these monoclonal antibodies flag the breast cancer cells and help the immune system to locate and destroy cancer cells more effectively16.
Monoclonal antibodies approved to treat breast cancer include:
- HER2-positive (HER2+) breast cancer: trastuzumab (Herceptin®), pertuzumab (Perjeta®), PHESGO® and margetuximab (Margenza®)
- Triple negative breast cancer (TNBC): pembrolizumab (keytruda®), atezolizumab (Tecentriq®)
Antibody-drug conjugates:
Antibody drug conjugates (ADCs) are made up of a monoclonal antibody and a chemotherapy drug joined by a chemical linker. The monoclonal antibody part of the ADC binds specifically to target antigens on cancer cells, enabling chemotherapy to be delivered and released directly at the site of tumour cells. This enables the ADC to function as a highly targeted drug that causes less side effects than chemotherapy that affects cells throughout the whole body.
The monoclonal antibody component of ADCs form an antigen-antibody complex upon binding to antigens on cancer cells. This means ADCs can act as immunotherapy, by flagging cancer cells to the immune system, leading to them being recognised and destroyed.
ADCs approved to treat breast cancer include:
- Trastuzumab emtansine (Kadcyla®) – approved to treat primary and secondary HER2+ breast cancer
- Trastuzumab deruxtecan (Enhertu®) – approved to treat secondary HER+ and secondary HER2-low (US and Europe only) breast cancer
- Sacituzumab govitecan (Trodevly®) – approved to treat secondary TNBC and secondary HR+ (US and Europe only) breast cancer
Have you tried tracking your treatment plan on OWise? With a range of treatment types to choose from, you can easily have your whole treatment plan at your fingertips. Simply enter the start and end date of each treatment and visualise your plan as a chart or list.
Potential benefits and challenges of immunotherapy
While immunotherapy is a new, exciting, and effective treatment option, it’s important to understand it may not be favourable for some patients. Immunotherapy has its challenges and benefits including:
Benefits18,10,17:
- Immunotherapy offers another treatment route when other methods, such as chemotherapy or radiotherapy fail.
- It can boost the effectiveness of other therapies, such as chemotherapy which may work better if you also receive immunotherapy.
- For some people there are fewer side effects associated with immunotherapies than other treatment options.
- Your immune system remembers targeting the cancer cells, meaning immunotherapy can reduce the risk of recurrence
- It is a targeted treatment, meaning it causes less harm to your surrounding normal tissues
- Immunotherapy can be used as a complementary therapy and works well with some other treatments to maximise results.
- There are ongoing clinical trials for many of the immunotherapies, providing patients access to experimental treatment options.
Challenges17,18:
- There is a possibility of a bad reaction. Where the IV drip or insertion area is could become bruised, painful, swollen, itchy or turn red.
- Associated side effects of immunotherapies include flu like symptoms (fever, chills, nausea, and fatigue), unexpected weight changes, swelling, diarrhoea, and heart palpitations.
- Immunotherapy does not work for everyone. Every immune system and response is slightly different, and only around half the people who take immunotherapies respond to it, or have a partial response.
- Your body can get used to immunotherapy, meaning it may become less responsible to treatment over time. Cancer cells can develop mechanisms to resist immunotherapy, leading to treatment resistance.
- As it is a fairly new category of treatment, the long-term side effects are still being studied and are relatively unknown.
- Immunotherapies, particularly the unique and personalised treatments can be fairly expensive in countries without national healthcare systems and may not be accessible for the majority.
- Immunotherapy can sometimes overstimulate the immune system, leading to autoimmune related side effects, which can sometimes be severe.
And that’s immunotherapy summed up
Immunotherapy is a promising avenue to treat breast cancer that boosts the body’s immune system to recognise and destroy cancer cells. There are passive and active immunotherapy treatments currently approved to treat breast cancer, and many ongoing trials into novel immunotherapy treatments.
At OWise, we want to make sure you are kept informed so make sure to follow our Instagram and Facebook for any updates. Any questions? Get in touch!
References:
- Shimu AS, Wei H, Li Q, Zheng X, Li B. 2022 Sep 15. The new progress in cancer immunotherapy. Clinical and Experimental Medicine. Doi: https://doi.org/10.1007/s10238-022-00887-0
- McComb S, Thiriot A, Akache B, Krishnan L, Stark F. 2019. Introduction to the Immune System. Methods in Molecular Biology (Clifton, NJ). 2024:1–24. doi: https://doi.org/10.1007/978-1-4939-9597-4_1 https://pubmed.ncbi.nlm.nih.gov/31364040/.
- Information NC for B, Pike USNL of M 8600 R, MD B, Usa 20894. 2020. The innate and adaptive immune systems. Institute for Quality and Efficiency in Health Care (IQWiG). https://www.ncbi.nlm.nih.gov/books/NBK279396/#:~:text=The%20innate%20immune%20system%20is.
- Cleveland Clinic. 2023. T-cells: Types and Function. Cleveland Clinic. [accessed 2023 Sep 12]. https://my.clevelandclinic.org/health/body/24630-t-cells#:~:text=T%2Dcells%20are%20a%20type.
- Kim SK, Cho SW. 2022. The Evasion Mechanisms of Cancer Immunity and Drug Intervention in the Tumor Microenvironment. Frontiers in Pharmacology. 13. doi: https://doi.org/10.3389/fphar.2022.868695.
- Pulendran B, van Driel R, Nossal GJV. 1997. Immunological tolerance in germinal centres. Immunology Today. 18(1):27–32. doi: https://doi.org/10.1016/s0167-5699(97)80011-4.
- Cancer Research UK. 2021 Jan 20. Types of immunotherapy| Cancer Research UK. wwwcancerresearchukorg. [accessed 2023 Sep 13]. https://www.cancerresearchuk.org/about-cancer/treatment/immunotherapy/types.
- Debien V, De Caluwé A, Wang X, Piccart-Gebhart M, Tuohy VK, Romano E, Buisseret L. 2023. Immunotherapy in breast cancer: an overview of current strategies and perspectives. npj Breast Cancer. 9(1). doi: https://doi.org/10.1038/s41523-023-00508-3
- Breastcancer.org. 2023 Sep 1. Immunotherapy Medicines Use Your Immune System To Attack Breast Cancer. wwwbreastcancerorg. [accessed 2023 Sep 13]. https://www.breastcancer.org/treatment/immunotherapy#.
- Yang Y-H, Liu J-W, Lu C, Wei J-F. 2022. CAR-T-cell Therapy for Breast Cancer: From Basic Research to Clinical Application. International Journal of Biological Sciences. 18(6):2609–2626. doi: https://doi.org/10.7150/ijbs.70120.
- Cancer.net. 2013 Sep 30. What are Cancer Vaccines? CancerNet. [accessed 2023 Sep 14]. https://www.cancer.net/navigating-cancer-care/how-cancer-treated/immunotherapy-and-vaccines/what-are-cancer-vaccines.
- Anassi E, Ndefo UA. 2011. Sipuleucel-T (Provenge) Injection. Pharmacy and Therapeutics. 36(4):197–202. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3086121/.
- Cancer Research UK. 2015 Mar 17. A trial looking at a vaccine for breast cancer with HER2 (PRESENT). Cancer Research UK. [accessed 2023 Sep 14]. https://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/a-trial-looking-vaccine-breast-cancer-her2-present#undefined.
- Cancer research UK. 2023 Jul 17. Targeted and immunotherapy drugs for breast cancer. wwwcancerresearchukorg. [accessed 2023 Sep 13]. https://www.cancerresearchuk.org/about-cancer/breast-cancer/treatment/targeted-immunotherapy-drugs.
- Behl A, Wani ZA, Das NN, Parmar VS, Len C, Malhotra S, Chhillar AK. 2023. Monoclonal antibodies in breast cancer: A critical appraisal. Critical Reviews in Oncology/Hematology. 183:103915. doi: https://doi.org/10.1016/j.critrevonc.2023.103915. [accessed 2023 Apr 19]. https://www.sciencedirect.com/science/article/abs/pii/S1040842823000033.
- American Cancer Society. 20 Dec 2023. Monoclonal antibodies and their side effects. American Cancer Society. [accesses 2024 May 10]. https://www.cancer.org/cancer/managing-cancer/treatment-types/immunotherapy/monoclonal-antibodies.html
- Booth S. 2021 Jan 21. Risks and Benefits of Immunotherapy. WebMD. [accessed 2023 Sep 14]. https://www.webmd.com/cancer/immunotherapy-risks-benefits.
- Dey A, Ghosh S, Jha S, Hazra S, Srivastava N, Chakraborty U, Roy AG. 2023. Recent advancement in breast cancer treatment using CAR T-cell therapy:- A review. Advances in Cancer Biology – Metastasis. 7:100090. doi: https://doi.org/10.1016/j.adcanc.2023.100090
