
Hormone therapy resistance: a scientific term that is rarely explained to the everyday reader. Although you may not know what it means, it is important to understand and recognise it. This is because hormone therapy resistance can occur in both primary breast cancer, up to 30% of women with primary breast cancer taking tamoxifen can become resistant, and occurs in the majority of women with hormone receptor positive secondary breast cancer1,2. It may be daunting, but knowing what to look out for and understanding the reason why your treatment plan may change is important in your treatment and side effect management.
To understand exactly what hormone therapy resistance is, firstly we need to discuss hormone therapy, then we can better understand why resistance takes place and look at what will happen if you become hormone therapy resistant.
If you are reading this, you most likely have hormone receptor positive breast cancer, also referred to as HR+, oestrogen/estrogen (British and American spelling, respectively) receptor positive, or ER+/PR+ breast cancer. This means that the breast cancer cells require hormones for growth. Specifically, they respond to the presence of oestrogen (ER) and progesterone (PR) in the blood, the major female sex hormones. As shown in the diagram, when oestrogen enters the breast cancer cell, it binds to receptors (think of the oestrogen being the key and the receptor the lock), activating them to bind to DNA and stimulate cell growth.
For more information about HR+ breast cancer, have a look at our blogs “What’ve hormones got to do with it: Early Stage or Secondary HR+ breast cancer”
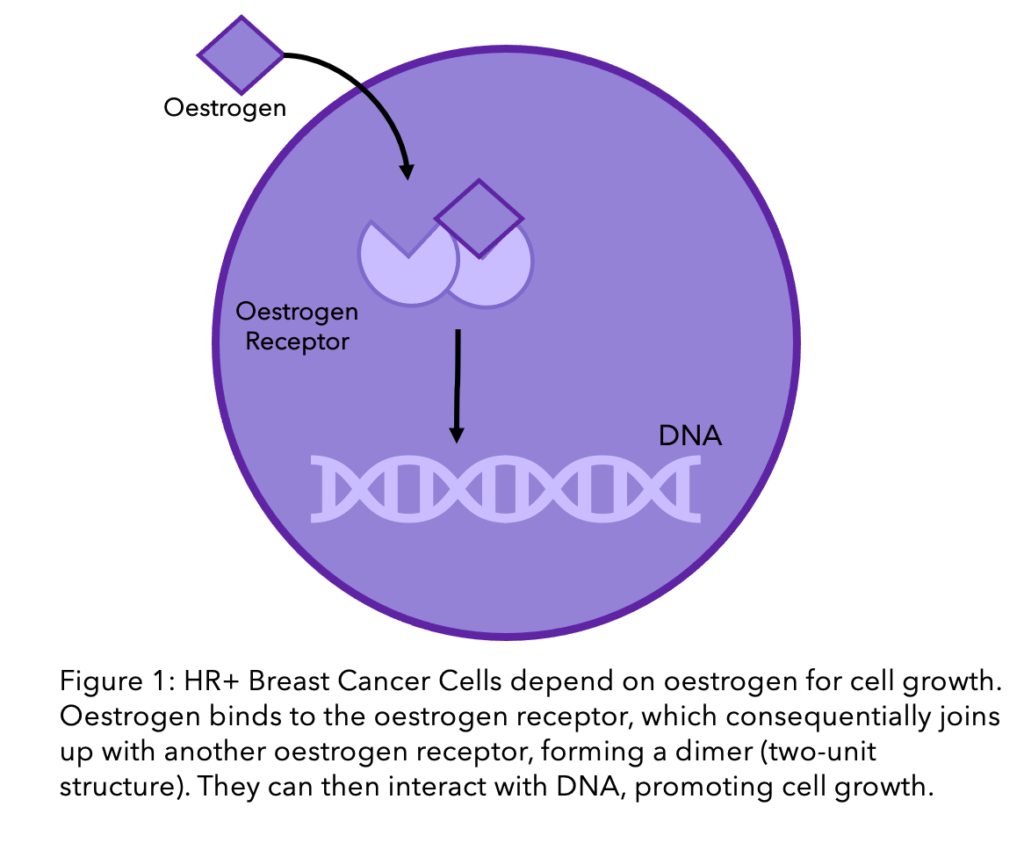
In the 1960’s HR+ breast cancer was first discovered, and this led to the development of hormone therapy treatment. This meant that we could use the breast cancers’ need of hormones to our advantage. By stopping the tumours access to hormones, the cancer cells can no longer grow, causing eventual cell death and stopping cancer in its tracks.
Did you know? The first hormone therapy to be discovered was tamoxifen, and it was actually made by mistake. A team of scientists in the 1960’s were looking into making an oral contraceptive that would suppress oestrogen. However, during testing, they found that tamoxifen stimulated, rather than suppressed, ovulation in women and could instead be used as a breast cancer treatment3.
Since the 1960’s a whole array of therapies targeting HR+ breast cancer have become available including aromatase inhibitors, fulvestrant, and more recently CDK4/6 inhibitors. Given the fact that nearly 70% of diagnosed breast cancers are HR+, these therapies have provided a successful treatment plan for many people4. However, whilst many HR+ patients respond well to hormone therapy up to 50%, and the majority of secondary HR+ breast cancers, may become resistant to treatment after a number of years1,2.
Enter hormone therapy resistance
So, what is it?
Hormone therapy resistance is when the cancer growth can no longer be stopped by hormone therapy. This means that even if you are receiving the medication, the cancer has found other ways to grow. Few people are thought to be resistant before starting therapy. Instead, the majority of hormone therapy resistance is acquired – meaning the breast cancer cells become resistant after exposure to the hormone therapy5. This resistance can result in the breast cancer treatments becoming less effective and increases the chance of the cancer coming back or spreading to other parts of the body. It is one of the major clinical problems which scientists are trying to resolve in breast cancer research.
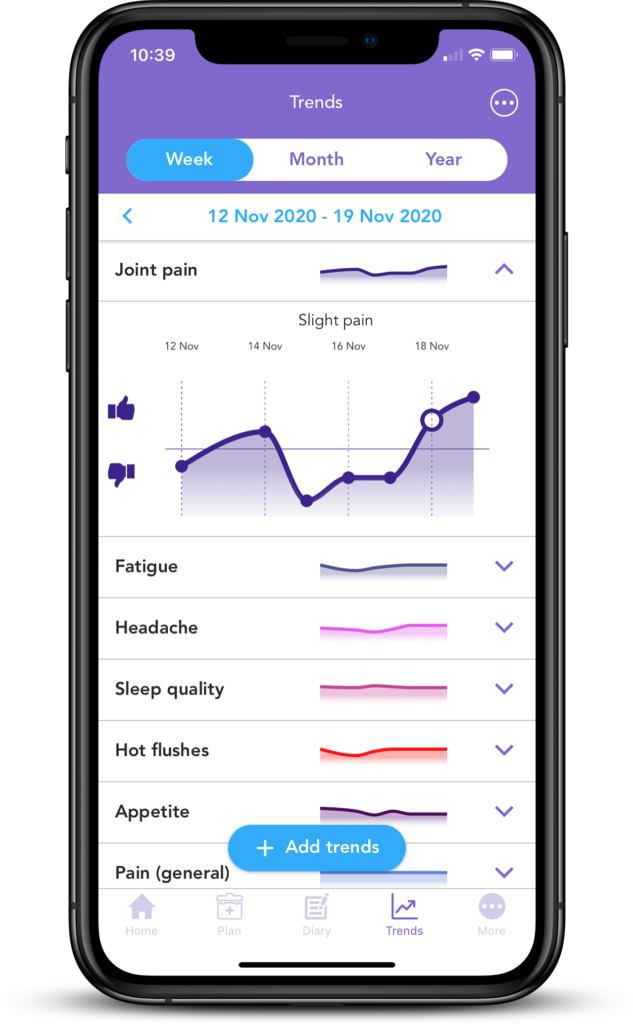 If you have primary or secondary HR+ breast cancer, you may want to document any changes in your symptoms and share these with your doctor. This can be used to both determine whether the treatment you are on is right for you and may help to indicate if that the treatment is not working. Most likely changes in symptoms won’t signify resistance, but sharing your symptoms or any concerns with your doctor may help you to rest assured that any signs of hormone therapy resistance will be noticed early on.
If you have primary or secondary HR+ breast cancer, you may want to document any changes in your symptoms and share these with your doctor. This can be used to both determine whether the treatment you are on is right for you and may help to indicate if that the treatment is not working. Most likely changes in symptoms won’t signify resistance, but sharing your symptoms or any concerns with your doctor may help you to rest assured that any signs of hormone therapy resistance will be noticed early on.
With OWise, you can track over 30 side effects and symptoms and visualise any changes in an easy-to-view graph. If you notice something isn’t quite right, you can send these trends straight to your care team. This can help them to understand your experience or any changes and decide on the next steps. You can download OWise, the multi-award winning and NHS accredited app, for free today.
What causes hormone therapy resistance?
One of the major issues related to hormone therapy resistance is the broad nature of how it can arise. There is not just one pathway that leads to resistance, instead there are multiple factors that in the right conditions can compound together. In this blog we will be looking at just one pathway of hormone therapy resistance. This is just a glimpse into the hormone resistance story, if you are interested in learning about other pathways, feel free to message us!
Oestrogen receptor
As explained earlier, oestrogen binds to a receptor in the cell, called the oestrogen receptor (ER). The ER is a protein, which is partially produced using the gene ESR1. A gene is a piece of genetic material (DNA) that provides instructions for the cell to create a protein. ESR1 is susceptible to changes, called mutations, which can lead to changes in the function and shape of the receptor and to it’s eventual resistance to treatment. Mutations in the ESR1 gene seem to accrue during increased exposure to hormone therapy. This could be one reason why mutations are less commonly seen in primary breast cancer than secondary5.
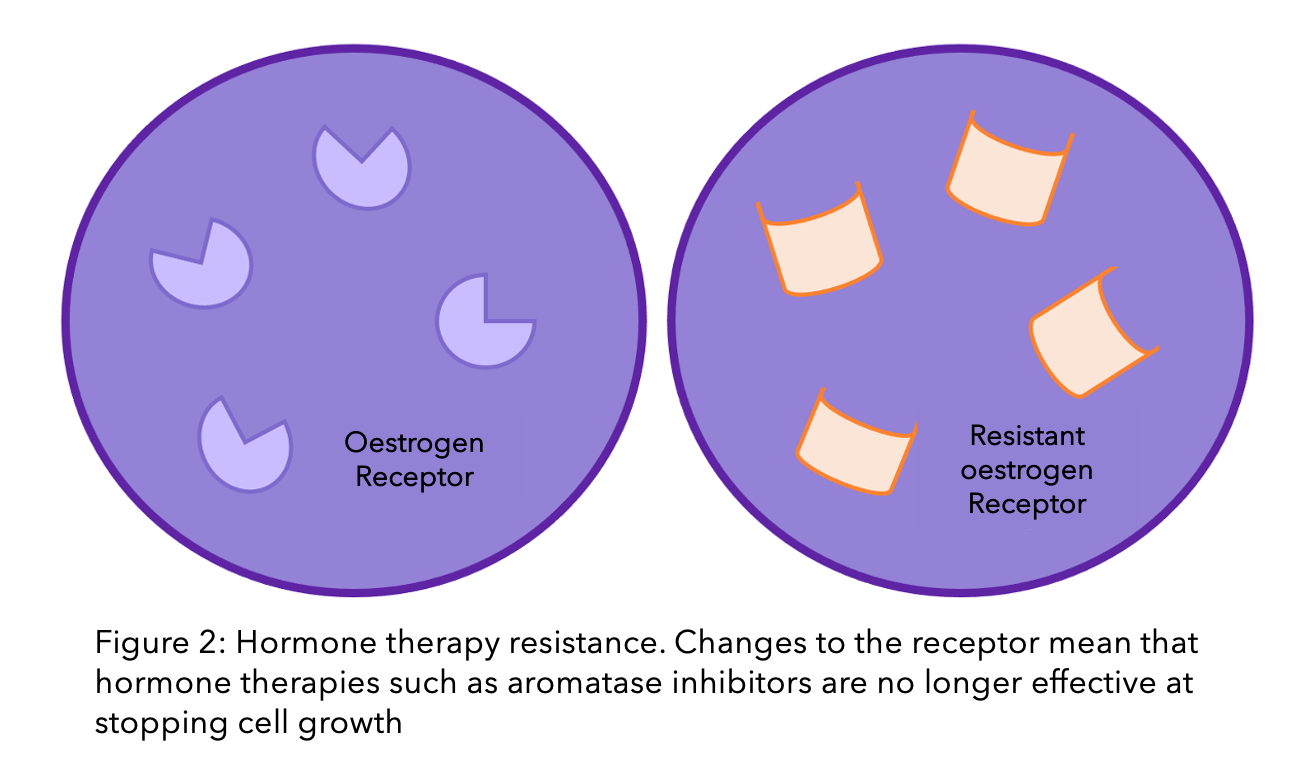
A study that collated research from lots of different teams found that in 24% of breast cancer cases, there was a mutation in the ESR1 gene5. In around half of these cases, there is not just one mutation, but multiple that all work together to resist treatment6,7.
But how does a genetic mutation cause resistance?
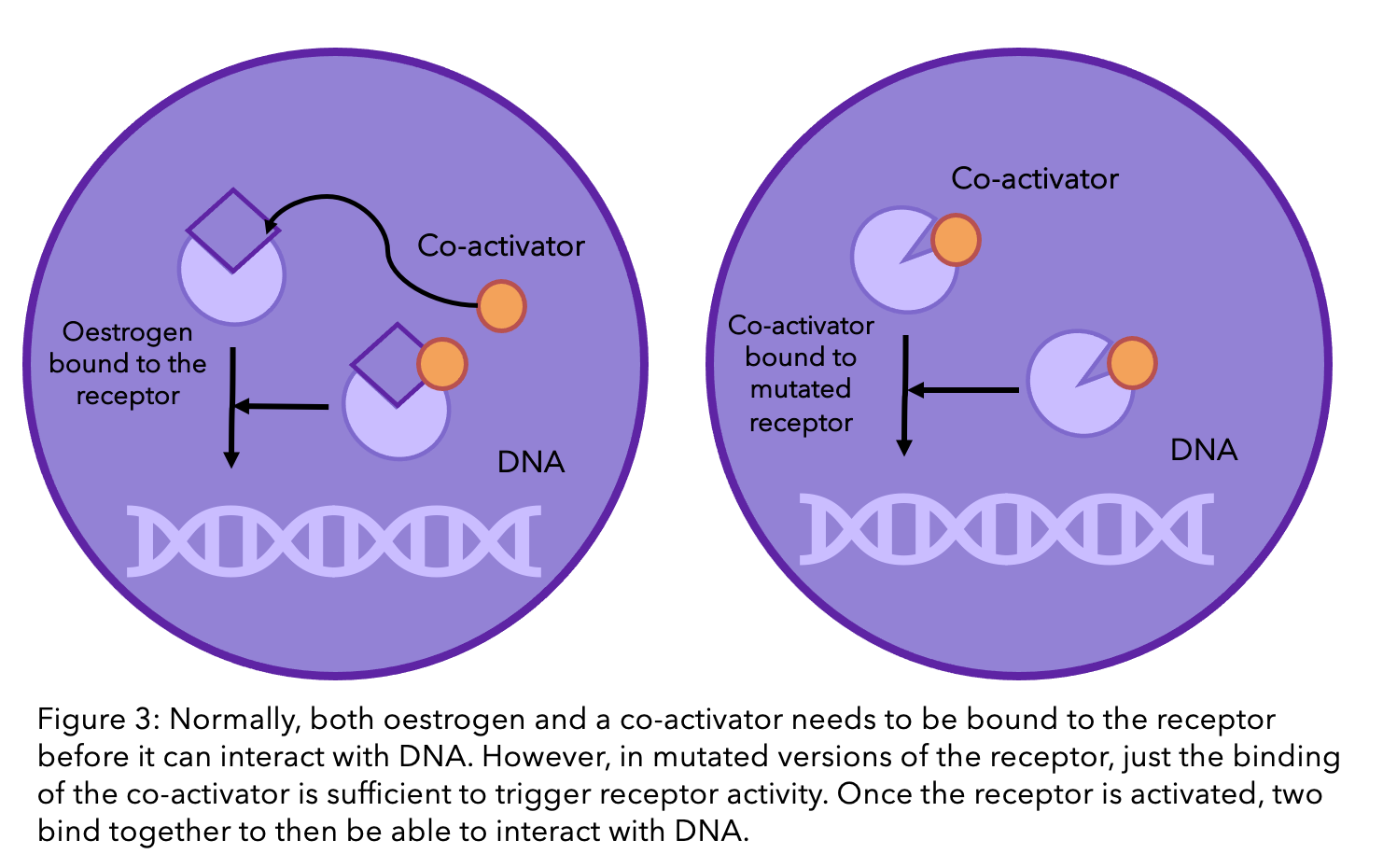
Normally, when oestrogen binds to the oestrogen receptor, there is one final step that is necessary in order for the receptor to be active and influence cell growth. This is when another molecule, called a “co-activator”, binds to the receptor and oestrogen (see diagram below). A current popular theory within the scientific community is that the mutations in the receptor means that the co-activator can bind to the receptor without the presence of oestrogen5. So even without oestrogen, cell growth can still be promoted.
Please note that drug resistance is a result of the nature of cancer and is not controlled by individual choices. With more research, drug resistance will be better understood and more effective treatments developed.
Are the oestrogen receptor (ER) mutations responsible for resistance to all hormone therapy drugs?
The simple answer is no. These mutations are the cause of resistance in some cases for the three key hormone therapy drugs, aromatase inhibitors, including letrozole (Famara®), anastrozole (Arimidex®) and exemestane (Aromasin®), tamoxifen and fulvestrant (Faslodex®). However, other drug resistance mechanisms that do not include the ER seem at play for each hormone treatment.
There is still a lot that we do not know, and further research is required to understand all the mechanisms of drug resistance. However, according to a recent study that collated the research from multiple teams, aromatase inhibitors (AIs) are the most susceptible to this form of ER resistance5. This is because the primary aim of aromatase inhibitors is to remove any oestrogen available to breast cancer cells (check out our previous blog explaining how AIs work). If the receptor no longer needs oestrogen to work, then this approach is ineffective. On top of this, ER mutations seem to occur predominantly when there is no circulating oestrogen5. Therefore, the action of the AIs (in removing circulating oestrogen) could itself lead to eventual resistance.
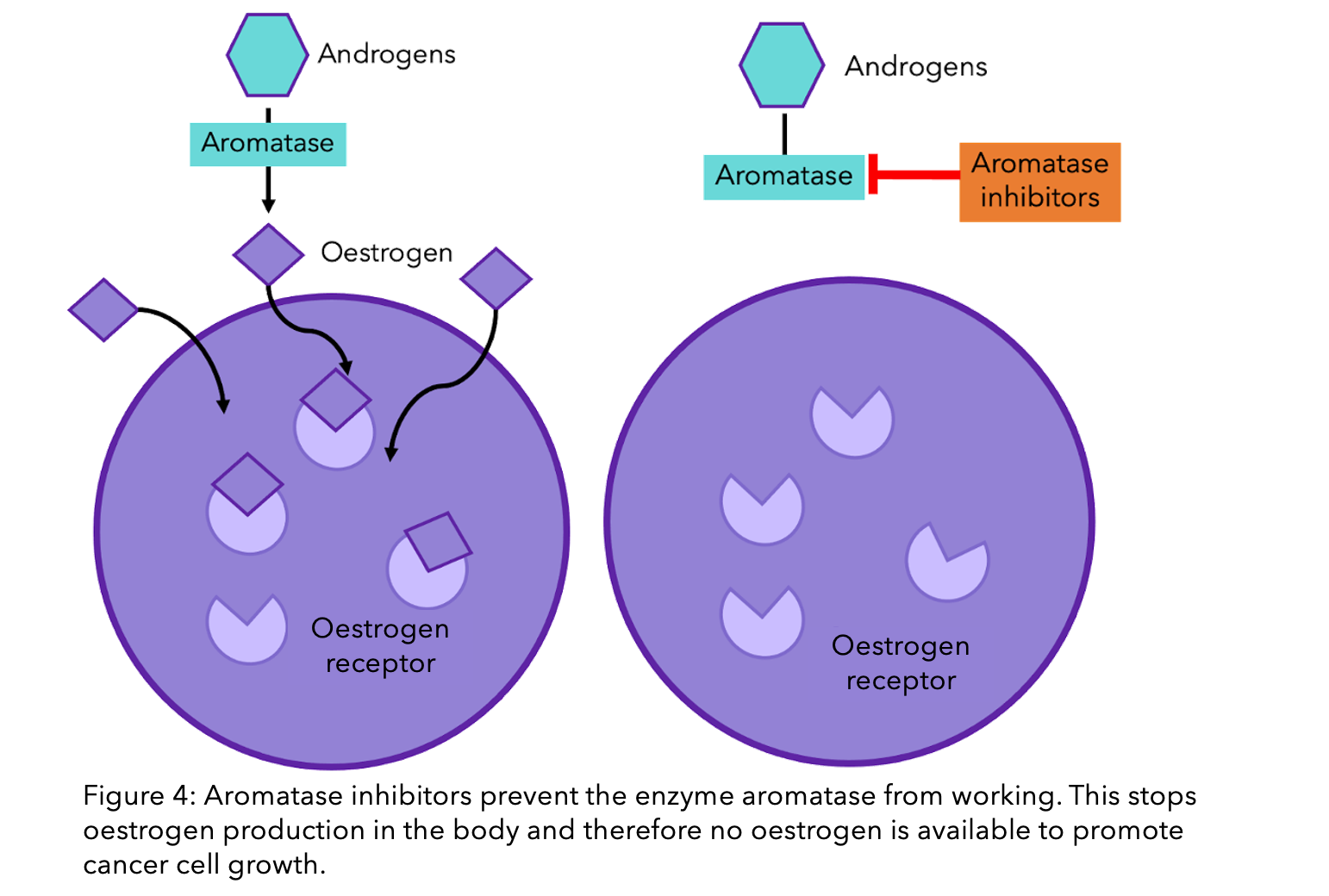
Tamoxifen and fulvestrant are a whole other story. Unlike AIs, these hormone drugs do not interact with oestrogen, but instead with the ER directly (check out our previous blog explaining how tamoxifen works). The ER’s ability to function without oestrogen does not necessarily alter the function of these drugs. However, if the mutation causes a change to the receptor’s shape, then these drugs may no longer be able to bind to the receptor and stop it’s function. This is because they have been designed to be the perfectly sized key to the ER’s lock (see Figure 1). If there is a slight alteration in the structure, the key can no longer fit inside the lock, let alone unlock it.
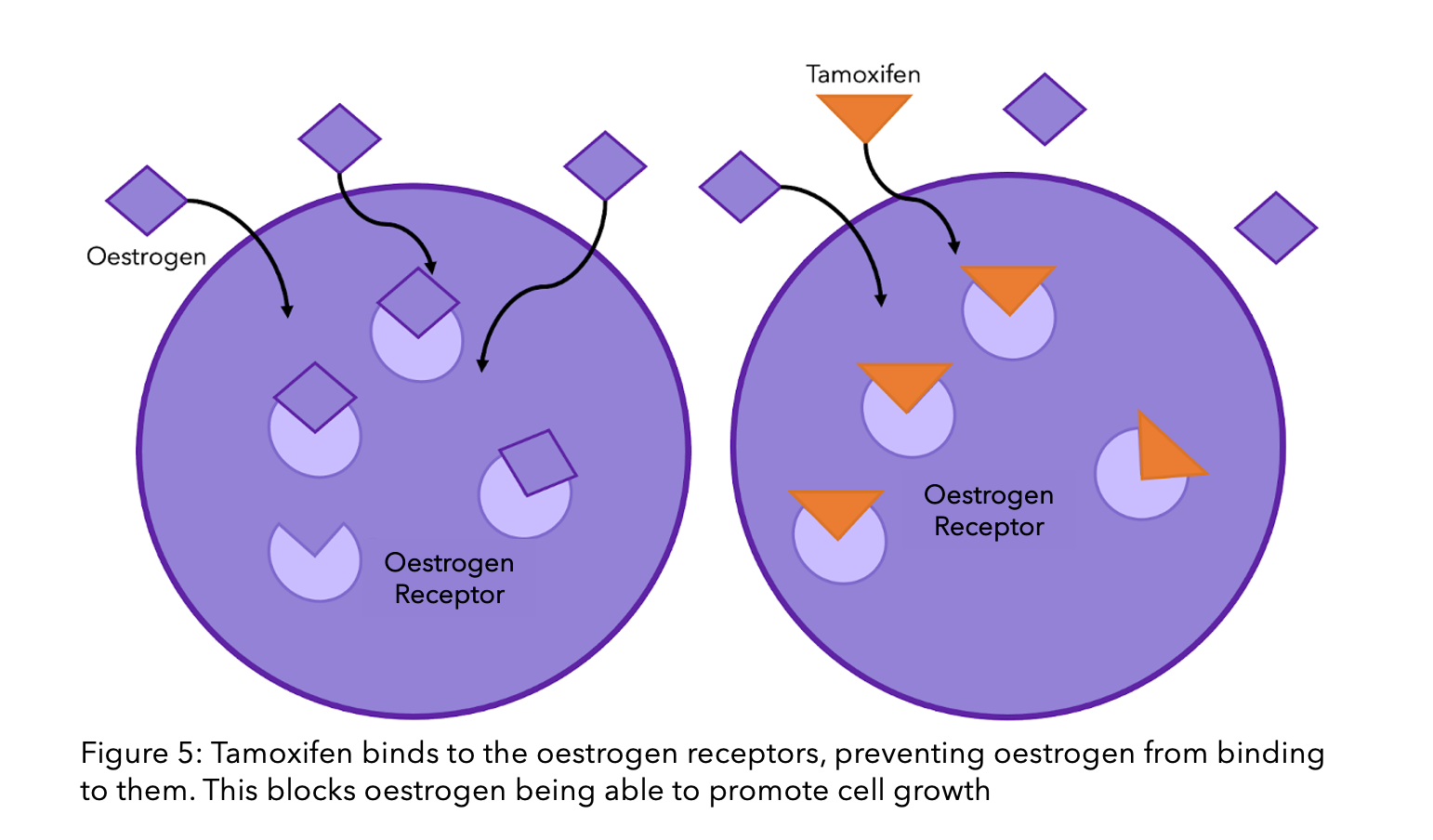
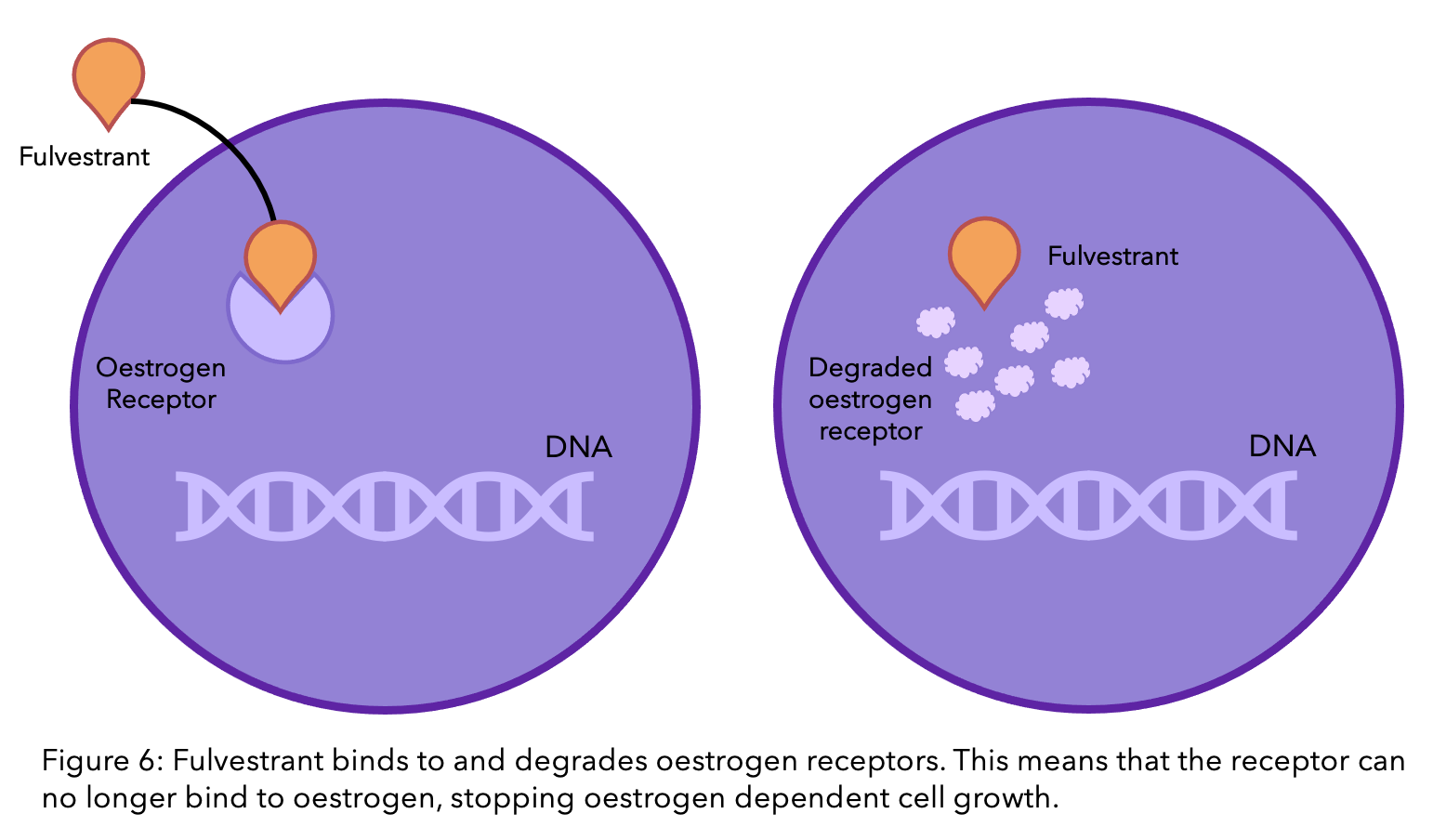
The story of resistance from tamoxifen and fulvestrant is a complicated, ever-changing one. It is estimated that one in three women with primary breast cancer who receive tamoxifen will acquire resistance within 15 years8. There are many theories, and many have been proven, however due to the complexity of the molecular interactions, there are still unanswered questions. There is a lot of ongoing research to find the mechanisms behind this process.
Did you know you can add all your treatments into OWise, creating one easy-to-view, interactive treatment plan?
What happens if you have hormone therapy resistance?
For primary breast cancer, hormone therapy resistance manifests as the cancer coming back during or after adjuvant endocrine therapy8. During your check ups for secondary breast cancer, tests will be done to see whether the cancer is responding (not changing or decreasing in size) to treatment. If the cancer is not responding, you will likely change therapies. This could be with a different hormone therapy, most likely fulvestrant (Faslodex®). At present, however, around 50 to 60% of women who do not respond to initial hormone therapy also do not respond to further hormone treatment9. Therefore, other treatments have been created to provide an alternative option that is effective in suppressing the growth of breast cancer.
A team at The Institute of Cancer Research have created a blood test that can detect ESR1 mutations in the blood. This will allow for quicker detection of potential resistance and help patients receive the most effective treatments quicker. Their findings need to be first reviewed before it can be implemented into the NHS10.
CDK4/6 inhibitors
CDK4/6 inhibitors are a class of oral drugs that inhibit proteins called Cyclin-Dependent Kinase (CDK) 4 and 6. These proteins are important in cell division. For cells to divide, they must go through a rigorously monitored cycle, partly controlled by CDK4 and CDK6. If these proteins are blocked, the cell cycle is interrupted and growth and division stopped11.
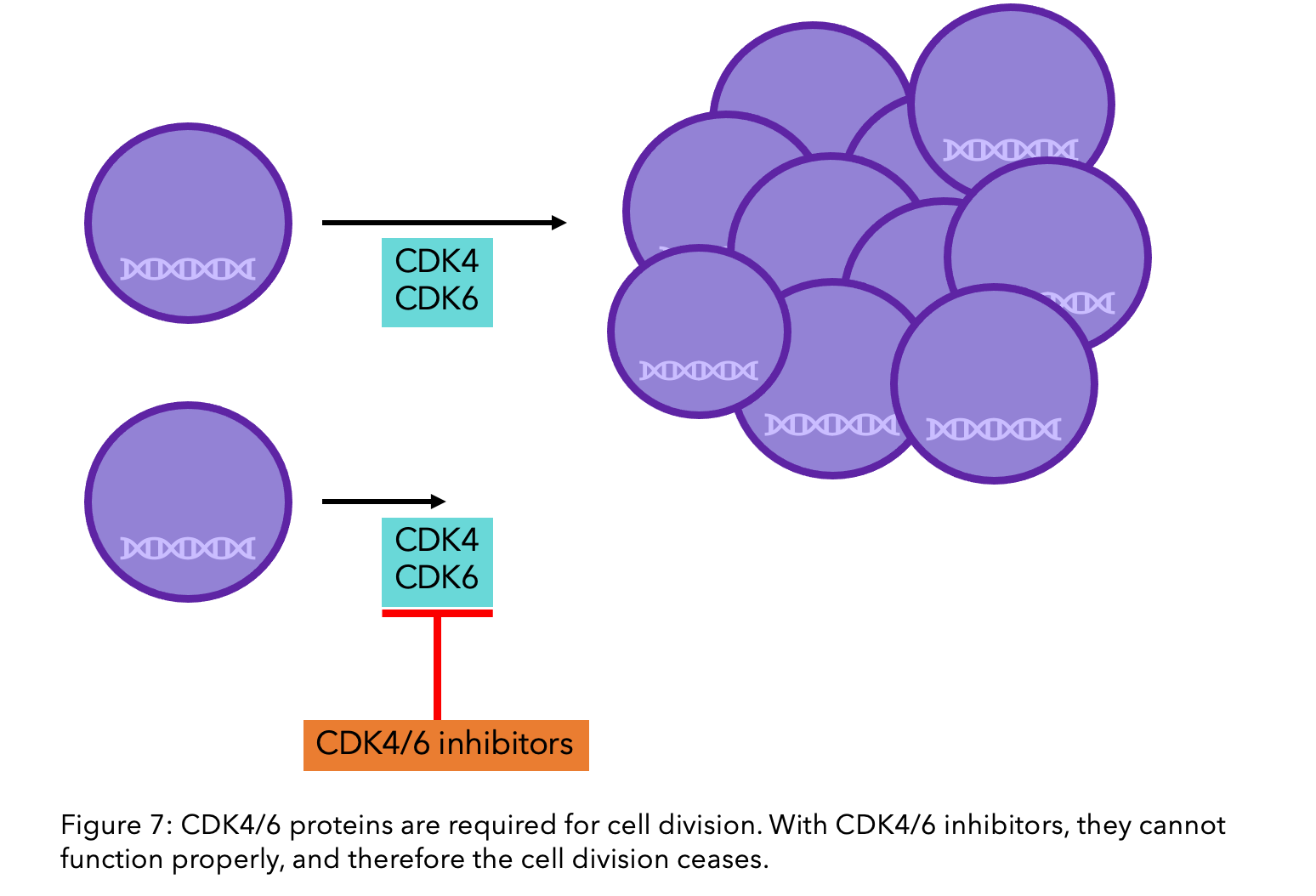
Research has shown that the use of CDK4/6 inhibitors can decrease the risk of fatality by 21% when patients are hormone resistant, and 27% when they are hormone sensitive12. If you have secondary cancer you could be offered CDK4/6 inhibitors through the NHS or the Cancer Drugs Fund13.
What is the Cancer Drugs Fund? The Cancer Drugs Fund is part of the British organisation that reviews new and existing drugs, called NICE. It was made to fund new drugs and accelerate the process of drugs becoming available in England. If there are recently developed treatments that may be of benefit to you, your specialist will fill out an application on your behalf. To find out more about Cancer Drugs Fund, head to Cancer Research UK’s website.
If you have not been previously treated for your secondary breast cancer, then you will be offered a CDK4/6 inhibitor, such as abemaciclib (Verzenio®), palbociclib (Ibrance®) or ribociclib (Kisqali®), in combination with an aromatase inhibitor as the first treatment. If you have already had hormone therapy that is no longer working, then you can apply to have a CDK4/6 inhibitor in combination with fulvestrant with the Cancer Drugs Fund. This is the case only if everolimus (Afinitor®) combined with exemestane (described below) is the other possible alternative14.
What is the difference between the available CDK4/6 inhibitors?
Abemaciclib is taken twice a day, continuously. Palbociclib and ribociclib are taken in cycles. First for 21 days daily, followed by 7 days off-treatment, before a 28-day cycle.
Side effects also differ between each. Abemaciclib is associated with increased incidence of diarrhea, palbociclib with increased incidence of neutropenia, as does ribociclib. The choice between the three will be decided between you and your doctor14.
Everolimus
Everolimus (Afinitor®) is a targeted therapy that blocks the protein mTOR, which, like CDK4/6, has responsibility for cell growth and division. The combination of everolimus with the aromatase inhibitor exemestane is recommended if other treatments such as hormone therapy are ineffective due to its success in increasing progression-free survival.
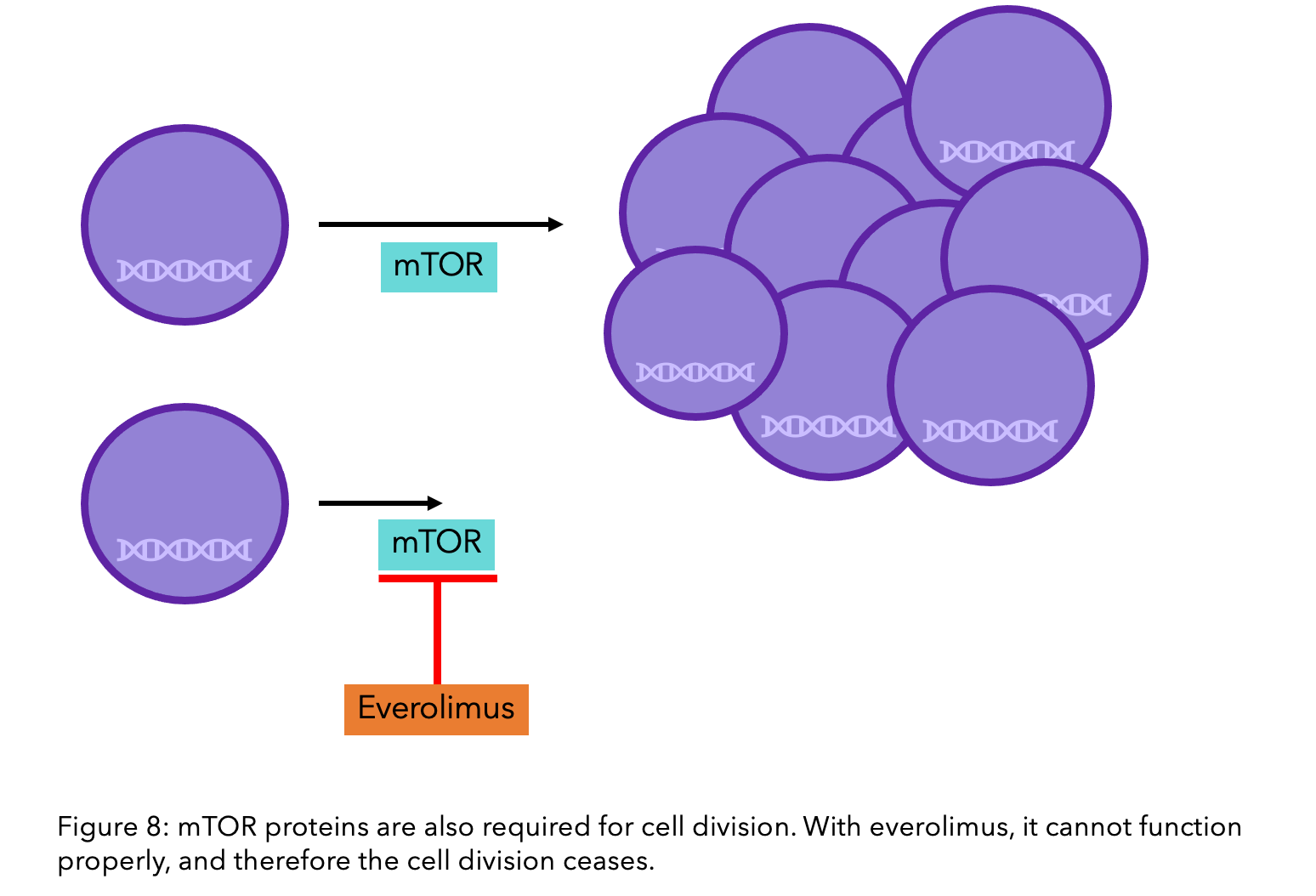
As with CDK4/6 inhibitors, there are no biomarkers that predict the response to everolimus, so it cannot be determined prior to treatment if it will be effective for your specific breast cancer15. Due to its more severe side effects, such as high blood sugar/diabetes, mouth sores and fatigue, CDK4/6 inhibitors alongside fulvestrant are the preferred treatment16.
If you experience adverse side effects when being treated with these life-extending drugs, be sure to monitor them with OWise. By sharing these with your doctor, they may be able to offer you additional treatments that stop these symptoms.
Advanced Genomic Profiling
If you are not responding to your current treatment, we suggest researching the potential of genomic profiling. This provides an in-depth understanding of your breast cancer type. Through rigorously searching your DNA, changes in genes are identified that could make you eligible for different treatments or clinical trials. Certain changes in genes can make you eligible for a specific treatment. Other findings are not as direct as these, and it is the accruing of various factors that will help decide the best treatment option for you. Although unavailable on the NHS at present, there are multiple companies that provide the service which can be refundable through healthcare insurance. These include Foundation Medicine, Guardant360, Claris and Tempus. It is vital to note that you should discuss this with your oncologist. Together you can decide whether it could improve your treatment.
I was diagnosed with ER+ (8/8) (score signifying how ER+ the breast cancer is – see pathology report) breast cancer at the age of 38, 4 years ago. After chemotherapy and radiotherapy, I started tamoxifen and Zoladex® to suppress my ovaries. About 1 and a half years after active treatment, I had my ovaries removed and swapped over to letrozole. At the end of 2020, I paid privately for genetic testing and the testing included my response to medications. Turns out, I’m a poor metaboliser of the gene that effects tamoxifen.
There is one thing that I know for sure, tamoxifen is not a fun drug to be on and if it wasn’t effective, I dealt with side effects I didn’t need to. Some people stop the drugs because it is hard on them. I didn’t. What can be done now to better understand why testing isn’t included before starting such a harsh drug that most people are on for 10 years?
– Karlee, from the Mommy Had a Little Cancer podcast (@mommyhadalittlecancer on Instagram)
We hope you enjoyed reading our blog and that with this knowledge, you better understand hormone therapy resistance and are aware of the possible implications to your treatment plan. It can be incredibly nerve-wracking not being confident in your breast cancer treatment and knowing whether or not it is working. Your routine scans will let you and your doctor know during your check-ups, but during the period between, it is important to keep track of any changes in your body and share these with your care team.
References
- P.G. Alluri, C. Speers, A.M. Chinnaiyan. (2014) Estrogen receptor mutations and their role in breast cancer progression. Breast Cancer Res., 16 (6).
- Musgrove EA, Sutherland R. (2009). Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer 9:631–43.
- Quirke V. (2017) Tamoxifen from Failed Contraceptive Pill to Best-Selling Breast Cancer Medicine: A Case-Study in Pharmaceutical Innovation. Frontiers in Pharmacology. 8
- Harvey, J. M., Clark, G. M., Osborne, C. K. & Allred, D. C. (1999) Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 17, 1474–1481
- Najim, O., et al. (2019) The association between type of endocrine therapy and development of estrogen receptor-1 mutation(s) in patients with hormone-sensitive advanced breast cancer: A systematic review and meta-analysis of randomized and non-randomized trials. Biochimica et Biophysica Acta (BBA) – Reviews on Cancer. 1872(2):188315.
- C. Fribbens, B. O’Leary, L. Kilburn, S. Hrebien, I. Garcia-Murillas, M. Beaney, et al. (2016) Plasma ESR1 mutations and the treatment of estrogen receptor-positive advanced breast Cancer J. Clin. Oncol., 34(25):2961-2968
- J.M. Spoerke, S. Gendreau, K. Walter, J.H. Qiu, T.R. Wilson, H. Savage, et al. (2016) Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat. Commun., 7
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). (2005) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365:1687–1717.
- NICE.org. (2012). Everolimus (Afinitor) in combination with exemestane for the treatment of advanced or metastatic HER2 negative, hormone receptor positive breast cancer after prior endocrine therapy. [online] Available at: https://www.nice.org.uk/guidance/ta295/documents/breast-cancer-her2-negative-oestrogen-receptor-positive-locally-advanced-or-metastatic-everolimus-with-an-aromatase-inhibitor-afinitor2
- Royal Marsden.nhs.uk. (2016)Blood test for new breast cancer subtype delays its progression. [online] Available at: https://www.royalmarsden.nhs.uk/news-and-events/news/blood-test-new-breast-cancer-subtype-delays-its-progression
- Breastcancer.org. (2020). CDK4/6 Inhibitors for Metastatic Breast Cancer: Ibrance, Kisqali, Verzenio. [online] Available at: https://www.breastcancer.org/treatment/targeted_therapies/cdk46-inhibitors
- Francesco Schettini, MD, Fabiola Giudici, MSPH, Mario Giuliano, MD, Massimo Cristofanilli, MD, Grazia Arpino, MD, Lucia Del Mastro, MD, Fabio Puglisi, MD, Sabino De Placido, MD, Ida Paris, MD, Pietro De Placido, MD, Sergio Venturini, PhD, Michelino De Laurentis, MD, PierFranco Conte, MD, Dejan Juric, MD, Antonio Llombart-Cussac, MD, Lajos Pusztai, MD, Aleix Prat, MD, Guy Jerusalem, MD, Angelo Di Leo, MD, Daniele Generali, MD. (2020) Overall Survival of CDK4/6-Inhibitor–Based Treatments in Clinically Relevant Subgroups of Metastatic Breast Cancer: Systematic Review and Meta-Analysis, JNCI: Journal of the National Cancer Institute. 112(11):1089–1097.
- pathways.nice.org.uk. (7AD). Advanced breast cancer – NICE Pathways. [online] Available at: https://pathways.nice.org.uk/pathways/advanced-breast-cancer [Accessed 07 January. 2021].
- Nice.org.uk. (2019) Abemaciclib with an aromatase inhibitor for previously untreated, hormone receptor-positive, HER2-negative, locally advanced or metastatic breast cancer. [online] Available at: https://www.nice.org.uk/guidance/ta563/chapter/3-Committee-discussion [Accessed 07 January. 2021].
- Baselga, J., Campone, M., Piccart, M., Burris, H.A., Rugo, H.S., Sahmoud, T., Noguchi, S., Gnant, M., Pritchard, K.I., Lebrun, F., Beck, J.T., Ito, Y., Yardley, D., Deleu, I., Perez, A., Bachelot, T., Vittori, L., Xu, Z., Mukhopadhyay, P., Lebwohl, D. and Hortobagyi, G.N. (2012). Everolimus in Postmenopausal Hormone-Receptor–Positive Advanced Breast Cancer. New England Journal of Medicine, 366(6), pp.520–529.
- Sledge, G.W., Toi, M., Neven, P., Sohn, J., Inoue, K., Pivot, X., Burdaeva, O., Okera, M., Masuda, N., Kaufman, P.A., Koh, H., Grischke, E.-M., Conte, P., Lu, Y., Barriga, S., Hurt, K., Frenzel, M., Johnston, S. and Llombart-Cussac, A. (2020). The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor–Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy—MONARCH 2. JAMA Oncology, 6(1), p.11
